Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study
- PMID: 25972273
- PMCID: PMC4430156
- DOI: 10.1136/bmj.h2298
Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study
Abstract
Objective: To evaluate maternal medical and perinatal outcomes associated with antipsychotic drug use in pregnancy.
Design: High dimensional propensity score (HDPS) matched cohort study.
Setting: Multiple linked population health administrative databases in the entire province of Ontario, Canada.
Participants: Among women who delivered a singleton infant between 2003 and 2012, and who were eligible for provincially funded drug coverage, those with ≥ 2 consecutive prescriptions for an antipsychotic medication during pregnancy, at least one of which was filled in the first or second trimester, were selected. Of these antipsychotic drug users, 1021 were matched 1:1 with 1021 non-users by means of a HDPS algorithm.
Main outcome measures: The main maternal medical outcomes were gestational diabetes, hypertensive disorders of pregnancy, and venous thromboembolism. The main perinatal outcomes were preterm birth (<37 weeks), and a birth weight <3rd or >97th centile. Conditional Poisson regression analysis was used to generate rate ratios and 95% confidence intervals, adjusting for additionally prescribed non-antipsychotic psychotropic medications.
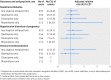
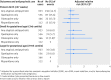
Results: Compared with non-users, women prescribed an antipsychotic medication in pregnancy did not seem to be at higher risk of gestational diabetes (rate ratio 1.10 (95% CI 0.77 to 1.57)), hypertensive disorders of pregnancy (1.12 (0.70 to 1.78)), or venous thromboembolism (0.95 (0.40 to 2.27)). The preterm birth rate, though high among antipsychotic users (14.5%) and matched non-users (14.3%), was not relatively different (rate ratio 0.99 (0.78 to 1.26)). Neither birth weight <3rd centile or >97th centile was associated with antipsychotic drug use in pregnancy (rate ratios 1.21 (0.81 to 1.82) and 1.26 (0.69 to 2.29) respectively).
Conclusions: Antipsychotic drug use in pregnancy had minimal evident impact on important maternal medical and short term perinatal outcomes. However, the rate of adverse outcomes is high enough to warrant careful assessment of maternal and fetal wellbeing among women prescribed an antipsychotic drug in pregnancy.
© Vigod et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Safety of psychotropic drugs in pregnancy.BMJ. 2015 May 13;350:h2260. doi: 10.1136/bmj.h2260. BMJ. 2015. PMID: 25972372 No abstract available.
References
-
- Vigod SN, Seeman MV, Ray JG, et al. Temporal trends in general and age-specific fertility rates among women with schizophrenia (1996-2009): a population-based study in Ontario, Canada. Schizophr Res 2012;139:169-75. - PubMed
-
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013;15:1-44. - PubMed
-
- Kennedy SH, Lam RW, Parikh SV, etal. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. Introduction. J Affect Disord 2009;117(suppl 1):S1-2. - PubMed
-
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry 2007;164:1817-24; quiz 923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
