Comparative Efficacy of Feline Leukemia Virus (FeLV) Inactivated Whole-Virus Vaccine and Canarypox Virus-Vectored Vaccine during Virulent FeLV Challenge and Immunosuppression
- PMID: 25972402
- PMCID: PMC4478526
- DOI: 10.1128/CVI.00034-15
Comparative Efficacy of Feline Leukemia Virus (FeLV) Inactivated Whole-Virus Vaccine and Canarypox Virus-Vectored Vaccine during Virulent FeLV Challenge and Immunosuppression
Abstract
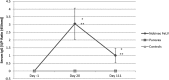
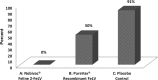
Four vaccines for feline leukemia virus (FeLV) are available in the United States. This study's purpose was to compare the efficacy of Nobivac feline 2-FeLV (an inactivated, adjuvanted whole-virus vaccine) and PureVax recombinant FeLV (a live, canarypox virus-vectored vaccine) following FeLV challenge. Cats were vaccinated at 9 and 12 weeks with Nobivac feline 2-FeLV (group A, n = 11) or PureVax recombinant FeLV (group B, n = 10). Group C (n = 11) comprised unvaccinated controls. At 3 months postvaccination, cats were immunosuppressed and challenged with FeLV-A/61E. The outcomes measured were persistent antigenemia at 12 weeks postchallenge (PC) and proviral DNA and viral RNA at 3 to 9 weeks PC. Persistent antigenemia was observed in 0 of 11 cats in group A, 5 of 10 cats in group B, and 10 of 11 cats in group C. Group A was significantly protected compared to those in groups B (P < 0.013) and C (P < 0.0001). No difference was found between groups B and C (P > 0.063). The preventable fraction was 100% for group A and 45% for group B. At 9 weeks PC, proviral DNA and viral RNA were detected 1 of 11 cats in group A, 6 of 10 cats in group B, and 9 of 11 cats in group C. Nucleic acid loads were significantly lower in group A than in group C (P < 0.01). Group A had significantly lower proviral DNA loads than group B at weeks 6 to 9 (P < 0.02). The viral RNA loads were significantly lower in group A than in group B at weeks 7 to 9 (P < 0.01). The results demonstrate that Nobivac feline 2-FeLV-vaccinated cats were fully protected against persistent antigenemia and had significantly smaller amounts of proviral DNA and plasma viral RNA loads than PureVax recombinant FeLV-vaccinated cats and unvaccinated controls.
Copyright © 2015, Patel et al.
Figures



Comment in
-
Immunosuppression in a Comparative Study of Feline Leukemia Virus Vaccines.Clin Vaccine Immunol. 2015 Dec;22(12):1294-5. doi: 10.1128/CVI.00497-15. Clin Vaccine Immunol. 2015. PMID: 26604264 Free PMC article. No abstract available.
-
Reply to "Immunosuppression in a Comparative Study of Feline Leukemia Virus Vaccines".Clin Vaccine Immunol. 2015 Dec;22(12):1296-7. doi: 10.1128/CVI.00504-15. Clin Vaccine Immunol. 2015. PMID: 26604265 Free PMC article. No abstract available.
References
-
- Hoover EA, Mullins JI. 1991. Feline leukemia virus infection and diseases. J Am Vet Med Assoc 199:1287–1297. - PubMed
-
- Levy JK, Crawford PC. 2005. Feline leukemia virus. In Ettinger SJ, Feldman EC (ed), Textbook of veterinary internal medicine, 6th ed WB Saunders, Philadelphia, PA.
-
- Rojko JL, Kociba GJ. 1991. Pathogenesis of infection by the feline leukemia virus. J Am Vet Med Assoc 199:1305–1310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

