Seroepidemiology of Human Papillomavirus 16 (HPV16) L2 and Generation of L2-Specific Human Chimeric Monoclonal Antibodies
- PMID: 25972404
- PMCID: PMC4478527
- DOI: 10.1128/CVI.00799-14
Seroepidemiology of Human Papillomavirus 16 (HPV16) L2 and Generation of L2-Specific Human Chimeric Monoclonal Antibodies
Abstract
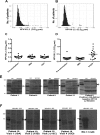
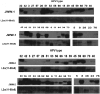
Presently, the seroprevalence of human papillomavirus (HPV) minor capsid antigen L2-reactive antibody is not well understood, and no serologic standard exists for L2-specific neutralizing antibodies. Therefore, we screened a total of 1,078 serum samples for HPV16 L2 reactivity, and these were obtained from four prior clinical studies: a population-based (n = 880) surveillance study with a high-risk HPV DNA prevalence of 10.8%, a cohort study of women (n = 160) with high-grade cervical intraepithelial neoplasia (CIN), and two phase II trials in women with high-grade vulvar intraepithelial neoplasia (VIN) receiving imiquimod therapy combined with either photodynamic therapy (PDT) (n = 19) or vaccination with a fusion protein comprising HPV16 L2, E7, and E6 (TA-CIN) (n = 19). Sera were screened sequentially by HPV16 L2 enzyme-linked immunosorbent assay (ELISA) and then Western blot. Seven of the 1,078 serum samples tested had L2-specific antibodies, but none were detectably neutralizing for HPV16. To develop a standard, we substituted human IgG1 sequences into conserved regions of two rodent monoclonal antibodies (MAbs) specific for neutralizing epitopes at HPV16 L2 residues 17 to 36 and 58 to 64, creating JWW-1 and JWW-2, respectively. These chimeric MAbs retained neutralizing activity and together reacted with 33/34 clinically relevant HPV types tested. In conclusion, our inability to identify an HPV16 L2-specific neutralizing antibody response even in the sera of patients with active genital HPV disease suggests the subdominance of L2 protective epitopes and the value of the chimeric MAbs JWW-1 and JWW-2 as standards for immunoassays to measure L2-specific human antibodies.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species.Cancer Res. 2006 Dec 1;66(23):11120-4. doi: 10.1158/0008-5472.CAN-06-2560. Cancer Res. 2006. PMID: 17145854
-
A novel HPV 16 L1-based chimeric virus-like particle containing E6 and E7 seroreactive epitopes permits highly specific detection of antibodies in patients with CIN 1 and HPV-16 infection.Virol J. 2011 Feb 9;8:59. doi: 10.1186/1743-422X-8-59. Virol J. 2011. PMID: 21306638 Free PMC article.
-
Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity.Vaccine. 2009 Feb 11;27(7):1040-9. doi: 10.1016/j.vaccine.2008.11.099. Epub 2008 Dec 16. Vaccine. 2009. PMID: 19095032 Free PMC article.
-
Mutation Profile of HPV16 L1 and L2 Genes in Different Geographic Areas.Viruses. 2022 Dec 31;15(1):141. doi: 10.3390/v15010141. Viruses. 2022. PMID: 36680181 Free PMC article. Review.
-
Recent Advances in Our Understanding of the Infectious Entry Pathway of Human Papillomavirus Type 16.Microorganisms. 2021 Oct 1;9(10):2076. doi: 10.3390/microorganisms9102076. Microorganisms. 2021. PMID: 34683397 Free PMC article. Review.
Cited by
-
Production and characterization of a novel HPV anti-L2 monoclonal antibody panel.Virology. 2018 Nov;524:106-113. doi: 10.1016/j.virol.2018.08.017. Epub 2018 Aug 28. Virology. 2018. PMID: 30170240 Free PMC article.
-
The second HPV serology meeting: Progress and challenges in standardization of human papillomavirus serology assays.Vaccine. 2023 Feb 3;41(6):1177-1181. doi: 10.1016/j.vaccine.2023.01.008. Epub 2023 Jan 13. Vaccine. 2023. PMID: 36642631 Free PMC article.
-
Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases.Vaccines (Basel). 2020 Oct 1;8(4):568. doi: 10.3390/vaccines8040568. Vaccines (Basel). 2020. PMID: 33019516 Free PMC article. Review.
-
Roles of Fc Domain and Exudation in L2 Antibody-Mediated Protection against Human Papillomavirus.J Virol. 2018 Jul 17;92(15):e00572-18. doi: 10.1128/JVI.00572-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29743371 Free PMC article.
-
Treatment and Prevention of HPV-Associated Skin Tumors by HPV Vaccination.Vaccines (Basel). 2024 Dec 20;12(12):1439. doi: 10.3390/vaccines12121439. Vaccines (Basel). 2024. PMID: 39772099 Free PMC article. Review.
References
-
- Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Munoz N. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. - DOI - PubMed
-
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, Pitisuttithum P, Restrepo JA, Stuart G, Woelber L, Yang YC, Cuzick J, Garland SM, Huh W, Kjaer SK, Bautista OM, Chan IS, Chen J, Gesser R, Moeller E, Ritter M, Vuocolo S, Luxembourg A, Broad Spectrum HPV Vaccine Study Group. 2015. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 372:711–723. doi:10.1056/NEJMoa1405044. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

