Rapid Whole-Genome Sequencing of Mycobacterium tuberculosis Isolates Directly from Clinical Samples
- PMID: 25972414
- PMCID: PMC4473240
- DOI: 10.1128/JCM.00486-15
Rapid Whole-Genome Sequencing of Mycobacterium tuberculosis Isolates Directly from Clinical Samples
Abstract
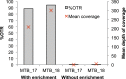
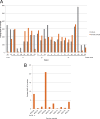
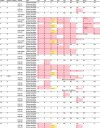
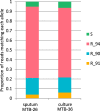
The rapid identification of antimicrobial resistance is essential for effective treatment of highly resistant Mycobacterium tuberculosis. Whole-genome sequencing provides comprehensive data on resistance mutations and strain typing for monitoring transmission, but unlike for conventional molecular tests, this has previously been achievable only from cultures of M. tuberculosis. Here we describe a method utilizing biotinylated RNA baits designed specifically for M. tuberculosis DNA to capture full M. tuberculosis genomes directly from infected sputum samples, allowing whole-genome sequencing without the requirement of culture. This was carried out on 24 smear-positive sputum samples, collected from the United Kingdom and Lithuania where a matched culture sample was available, and 2 samples that had failed to grow in culture. M. tuberculosis sequencing data were obtained directly from all 24 smear-positive culture-positive sputa, of which 20 were of high quality (>20× depth and >90% of the genome covered). Results were compared with those of conventional molecular and culture-based methods, and high levels of concordance between phenotypical resistance and predicted resistance based on genotype were observed. High-quality sequence data were obtained from one smear-positive culture-negative case. This study demonstrated for the first time the successful and accurate sequencing of M. tuberculosis genomes directly from uncultured sputa. Identification of known resistance mutations within a week of sample receipt offers the prospect for personalized rather than empirical treatment of drug-resistant tuberculosis, including the use of antimicrobial-sparing regimens, leading to improved outcomes.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D'Ambrosio L, Zignol M, Floyd K, Centis R, Cirillo DM, Tortoli E, Gilpin C, de Dieu Iragena J, Falzon D, Raviglione M. 2013. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J 42:252–271. doi:10.1183/09031936.00157212. - DOI - PubMed
-
- Drobniewski F, Nikolayevskyy V, Maxeiner H, Balabanova Y, Casali N, Kontsevaya I, Ignatyeva O. 2013. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med 11:190. doi:10.1186/1741-7015-11-190. - DOI - PMC - PubMed
-
- Casali N, Nikolayevskyy V, Balabanova Y, Harris SR, Ignatyeva O, Kontsevaya I, Corander J, Bryant J, Parkhill J, Nejentsev S, Horstmann RD, Brown T, Drobniewski F. 2014. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat Genet 46:279–286. doi:10.1038/ng.2878. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

