Cryptococcal heat shock protein 70 homolog Ssa1 contributes to pulmonary expansion of Cryptococcus neoformans during the afferent phase of the immune response by promoting macrophage M2 polarization
- PMID: 25972480
- PMCID: PMC4458402
- DOI: 10.4049/jimmunol.1402719
Cryptococcal heat shock protein 70 homolog Ssa1 contributes to pulmonary expansion of Cryptococcus neoformans during the afferent phase of the immune response by promoting macrophage M2 polarization
Abstract
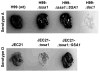
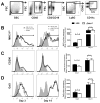
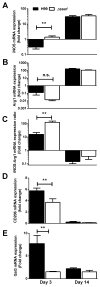
Numerous virulence factors expressed by Cryptococcus neoformans modulate host defenses by promoting nonprotective Th2-biased adaptive immune responses. Prior studies demonstrate that the heat shock protein 70 homolog, Ssa1, significantly contributes to serotype D C. neoformans virulence through the induction of laccase, a Th2-skewing and CNS tropic factor. In the present study, we sought to determine whether Ssa1 modulates host defenses in mice infected with a highly virulent serotype A strain of C. neoformans (H99). To investigate this, we assessed pulmonary fungal growth, CNS dissemination, and survival in mice infected with either H99, an SSA1-deleted H99 strain (Δssa1), and a complement strain with restored SSA1 expression (Δssa1::SSA1). Mice infected with the Δssa1 strain displayed substantial reductions in lung fungal burden during the innate phase (days 3 and 7) of the host response, whereas less pronounced reductions were observed during the adaptive phase (day 14) and mouse survival increased only by 5 d. Surprisingly, laccase activity assays revealed that Δssa1 was not laccase deficient, demonstrating that H99 does not require Ssa1 for laccase expression, which explains the CNS tropism we still observed in the Ssa1-deficient strain. Lastly, our immunophenotyping studies showed that Ssa1 directly promotes early M2 skewing of lung mononuclear phagocytes during the innate phase, but not the adaptive phase, of the immune response. We conclude that Ssa1's virulence mechanism in H99 is distinct and laccase-independent. Ssa1 directly interferes with early macrophage polarization, limiting innate control of C. neoformans, but ultimately has no effect on cryptococcal control by adaptive immunity.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures

References
-
- Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. The New England journal of medicine. 1989;321:794–799. - PubMed
-
- Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, Henderson H, Kauffman CA, Haas DW, Saccente M, Hamill RJ, Holloway MS, Warren RM, Dismukes WE. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2001;33:690–699. - PubMed
-
- Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. Aids. 2007;21:2119–2129. - PubMed
-
- Huffnagle GB, Lipscomb MF. Cells and cytokines in pulmonary cryptococcosis. Research in immunology. 1998;149:387–396. discussion 512–384. - PubMed
-
- Huffnagle GB, Toews GB, Burdick MD, Boyd MB, McAllister KS, McDonald RA, Kunkel SL, Strieter RM. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

