Measles Virus Defective Interfering RNAs Are Generated Frequently and Early in the Absence of C Protein and Can Be Destabilized by Adenosine Deaminase Acting on RNA-1-Like Hypermutations
- PMID: 25972541
- PMCID: PMC4505647
- DOI: 10.1128/JVI.01017-15
Measles Virus Defective Interfering RNAs Are Generated Frequently and Early in the Absence of C Protein and Can Be Destabilized by Adenosine Deaminase Acting on RNA-1-Like Hypermutations
Abstract
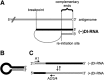
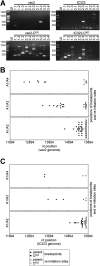
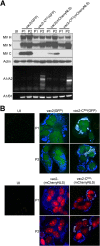
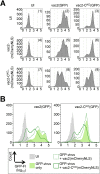
Defective interfering RNAs (DI-RNAs) of the viral genome can form during infections of negative-strand RNA viruses and outgrow full-length viral genomes, thereby modulating the severity and duration of infection. Here we document the frequent de novo generation of copy-back DI-RNAs from independent rescue events both for a vaccine measles virus (vac2) and for a wild-type measles virus (IC323) as early as passage 1 after virus rescue. Moreover, vaccine and wild-type C-protein-deficient (C-protein-knockout [CKO]) measles viruses generated about 10 times more DI-RNAs than parental virus, suggesting that C enhances the processivity of the viral polymerase. We obtained the nucleotide sequences of 65 individual DI-RNAs, identified breakpoints and reinitiation sites, and predicted their structural features. Several DI-RNAs possessed clusters of A-to-G or U-to-C transitions. Sequences flanking these mutation sites were characteristic of those favored by adenosine deaminase acting on RNA-1 (ADAR1), which catalyzes in double-stranded RNA the C-6 deamination of adenosine to produce inosine, which is recognized as guanosine, a process known as A-to-I RNA editing. In individual DI-RNAs the transitions were of the same type and occurred on both sides of the breakpoint. These patterns of mutations suggest that ADAR1 edits unencapsidated DI-RNAs that form double-strand RNA structures. Encapsidated DI-RNAs were incorporated into virus particles, which reduced the infectivity of virus stocks. The CKO phenotype was dominant: DI-RNAs derived from vac2 with a CKO suppressed the replication of vac2, as shown by coinfections of interferon-incompetent lymphatic cells with viruses expressing different fluorescent reporter proteins. In contrast, coinfection with a C-protein-expressing virus did not counteract the suppressive phenotype of DI-RNAs.
Importance: Recombinant measles viruses (MVs) are in clinical trials as cancer therapeutics and as vectored vaccines for HIV-AIDS and other infectious diseases. The efficacy of MV-based vectors depends on their replication proficiency and immune activation capacity. Here we document that copy-back defective interfering RNAs (DI-RNAs) are generated by recombinant vaccine and wild-type MVs immediately after rescue. The MV C protein interferes with DI-RNA generation and may enhance the processivity of the viral polymerase. We frequently detected clusters of A-to-G or U-to-C transitions and noted that sequences flanking individual mutations contain motifs favoring recognition by the adenosine deaminase acting on RNA-1 (ADAR1). The consistent type of transitions on the DI-RNAs indicates that these are direct substrates for editing by ADAR1. The ADAR1-mediated biased hypermutation events are consistent with the protein kinase R (PKR)-ADAR1 balancing model of innate immunity activation. We show by coinfection that the C-defective phenotype is dominant.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Lamb RA, Parks GD. 2013. Paramyxoviridae, p 957–995. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Griffin DE. 2013. Measles virus, p 1042–1069. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

