Bile Acids Reduce Prion Conversion, Reduce Neuronal Loss, and Prolong Male Survival in Models of Prion Disease
- PMID: 25972546
- PMCID: PMC4505631
- DOI: 10.1128/JVI.01165-15
Bile Acids Reduce Prion Conversion, Reduce Neuronal Loss, and Prolong Male Survival in Models of Prion Disease
Abstract
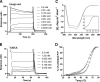
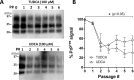
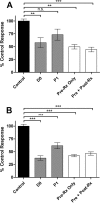
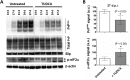
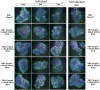
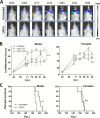
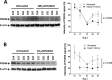
Prion diseases are fatal neurodegenerative disorders associated with the conversion of cellular prion protein (PrPC) into its aberrant infectious form (PrPSc). There is no treatment available for these diseases. The bile acids tauroursodeoxycholic acid(TUDCA) and ursodeoxycholic acid (UDCA) have been recently shown to be neuroprotective in other protein misfolding disease models, including Parkinson’s, Huntington’s and Alzheimer’s diseases, and also in humans with amyotrophic lateral sclerosis.Here, we studied the therapeutic efficacy of these compounds in prion disease. We demonstrated that TUDCA and UDCA substantially reduced PrP conversion in cell-free aggregation assays, as well as in chronically and acutely infected cell cultures. This effect was mediated through reduction of PrPSc seeding ability, rather than an effect on PrPC. We also demonstrated the ability of TUDCA and UDCA to reduce neuronal loss in prion-infected cerebellar slice cultures. UDCA treatment reduced astrocytosis and prolonged survival in RML prion-infected mice. Interestingly, these effects were limited to the males, implying a gender-specific difference in drug metabolism. Beyond effects on PrPSc, we found that levels of phosphorylated eIF2 were increased at early time points, with correlated reductions in postsynaptic density protein 95. As demonstrated for other neurodegenerative diseases, we now show that TUDCA and UDCA may have a therapeutic role in prion diseases, with effects on both prion conversion and neuroprotection. Our findings, together with the fact that these natural compounds are orally bioavailable, permeable to the blood-brain barrier, and U.S. Food and Drug Administration-approved for use in humans, make these compounds promising alternatives for the treatment of prion diseases.
Importance: Prion diseases are fatal neurodegenerative diseases that are transmissible to humans and other mammals. There are no disease-modifying therapies available, despite decades of research. Treatment targets have included inhibition of protein accumulation,clearance of toxic aggregates, and prevention of downstream neurodegeneration. No one target may be sufficient; rather, compounds which have a multimodal mechanism, acting on different targets, would be ideal. TUDCA and UDCA are bile acids that may fulfill this dual role. Previous studies have demonstrated their neuroprotective effects in several neurodegenerative disease models, and we now demonstrate that this effect occurs in prion disease, with an added mechanistic target of upstream prion seeding. Importantly, these are natural compounds which are orally bioavailable, permeable to the blood-brain barrier, and U.S.Food and Drug Administration-approved for use in humans with primary biliary cirrhosis. They have recently been proven efficacious in human amyotrophic lateral sclerosis. Therefore, these compounds are promising options for the treatment of prion diseases.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

