Graded boosting of synaptic signals by low-threshold voltage-activated calcium conductance
- PMID: 25972583
- PMCID: PMC4507965
- DOI: 10.1152/jn.00170.2015
Graded boosting of synaptic signals by low-threshold voltage-activated calcium conductance
Abstract
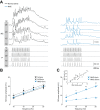
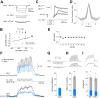
Low-threshold voltage-activated calcium conductances (LT-VACCs) play a substantial role in shaping the electrophysiological attributes of neurites. We have investigated how these conductances affect synaptic integration in a premotor nonspiking (NS) neuron of the leech nervous system. These cells exhibit an extensive neuritic tree, do not fire Na(+)-dependent spikes, but express an LT-VACC that was sensitive to 250 μM Ni(2+) and 100 μM NNC 55-0396 (NNC). NS neurons responded to excitation of mechanosensory pressure neurons with depolarizing responses for which amplitude was a linear function of the presynaptic firing frequency. NNC decreased these synaptic responses and abolished the concomitant widespread Ca(2+) signals. Coherent with the interpretation that the LT-VACC amplified signals at the postsynaptic level, this conductance also amplified the responses of NS neurons to direct injection of sinusoidal current. Synaptic amplification thus is achieved via a positive feedback in which depolarizing signals activate an LT-VACC that, in turn, boosts these signals. The wide distribution of LT-VACC could support the active propagation of depolarizing signals, turning the complex NS neuritic tree into a relatively compact electrical compartment.
Keywords: calcium conductance; dendritic integration; nonspiking; synaptic amplification; window current.
Copyright © 2015 the American Physiological Society.
Figures



Similar articles
-
Wide propagation of graded signals in nonspiking neurons.J Neurophysiol. 2013 Feb;109(3):711-20. doi: 10.1152/jn.00934.2012. Epub 2012 Nov 14. J Neurophysiol. 2013. PMID: 23155168
-
Calcium spikes in a leech nonspiking neuron.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009 Feb;195(2):139-50. doi: 10.1007/s00359-008-0393-4. Epub 2008 Nov 26. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009. PMID: 19034463
-
Rebound excitation triggered by synaptic inhibition in cerebellar nuclear neurons is suppressed by selective T-type calcium channel block.J Neurophysiol. 2011 Nov;106(5):2653-61. doi: 10.1152/jn.00612.2011. Epub 2011 Aug 17. J Neurophysiol. 2011. PMID: 21849607
-
Synaptic integration in dendritic trees.J Neurobiol. 2005 Jul;64(1):75-90. doi: 10.1002/neu.20144. J Neurobiol. 2005. PMID: 15884003 Review.
-
Low-threshold calcium currents in central nervous system neurons.Annu Rev Physiol. 1996;58:329-48. doi: 10.1146/annurev.ph.58.030196.001553. Annu Rev Physiol. 1996. PMID: 8815798 Review.
Cited by
-
9-Phenanthrol modulates postinhibitory rebound and afterhyperpolarizing potentials in an excitatory motor neuron of the medicinal leech.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017 Aug;203(8):613-633. doi: 10.1007/s00359-017-1178-4. Epub 2017 May 11. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017. PMID: 28497254
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

