Properties of precise firing synchrony between synaptically coupled cortical interneurons depend on their mode of coupling
- PMID: 25972585
- PMCID: PMC4509386
- DOI: 10.1152/jn.00304.2015
Properties of precise firing synchrony between synaptically coupled cortical interneurons depend on their mode of coupling
Abstract
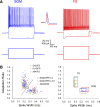
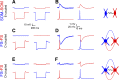
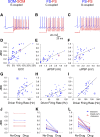
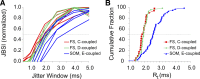
Precise spike synchrony has been widely reported in the central nervous system, but its functional role in encoding, processing, and transmitting information is yet unresolved. Of particular interest is firing synchrony between inhibitory cortical interneurons, thought to drive various cortical rhythms such as gamma oscillations, the hallmark of cognitive states. Precise synchrony can arise between two interneurons connected electrically, through gap junctions, chemically, through fast inhibitory synapses, or dually, through both types of connections, but the properties of synchrony generated by these different modes of connectivity have never been compared in the same data set. In the present study we recorded in vitro from 152 homotypic pairs of two major subtypes of mouse neocortical interneurons: parvalbumin-containing, fast-spiking (FS) interneurons and somatostatin-containing (SOM) interneurons. We tested firing synchrony when the two neurons were driven to fire by long, depolarizing current steps and used a novel synchrony index to quantify the strength of synchrony, its temporal precision, and its dependence on firing rate. We found that SOM-SOM synchrony, driven solely by electrical coupling, was less precise than FS-FS synchrony, driven by inhibitory or dual coupling. Unlike SOM-SOM synchrony, FS-FS synchrony was strongly firing rate dependent and was not evident at the prototypical 40-Hz gamma frequency. Computer simulations reproduced these differences in synchrony without assuming any differences in intrinsic properties, suggesting that the mode of coupling is more important than the interneuron subtype. Our results provide novel insights into the mechanisms and properties of interneuron synchrony and point out important caveats in current models of cortical oscillations.
Keywords: cortical interneurons; electrical coupling; firing synchrony; gap junctions; temporal precision; unitary IPSP.
Copyright © 2015 the American Physiological Society.
Figures


Similar articles
-
Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically.J Neurosci. 2011 Mar 2;31(9):3351-61. doi: 10.1523/JNEUROSCI.4881-10.2011. J Neurosci. 2011. PMID: 21368047 Free PMC article.
-
Differential Excitation of Distally versus Proximally Targeting Cortical Interneurons by Unitary Thalamocortical Bursts.J Neurosci. 2016 Jun 29;36(26):6906-16. doi: 10.1523/JNEUROSCI.0739-16.2016. J Neurosci. 2016. PMID: 27358449 Free PMC article.
-
Excitatory Inputs Determine Phase-Locking Strength and Spike-Timing of CA1 Stratum Oriens/Alveus Parvalbumin and Somatostatin Interneurons during Intrinsically Generated Hippocampal Theta Rhythm.J Neurosci. 2016 Jun 22;36(25):6605-22. doi: 10.1523/JNEUROSCI.3951-13.2016. J Neurosci. 2016. PMID: 27335395 Free PMC article.
-
Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex.J Neurocytol. 2002 Mar-Jun;31(3-5):277-87. doi: 10.1023/a:1024126110356. J Neurocytol. 2002. PMID: 12815247 Review.
-
Connexon connexions in the thalamocortical system.Prog Brain Res. 2005;149:41-57. doi: 10.1016/S0079-6123(05)49004-4. Prog Brain Res. 2005. PMID: 16226575 Review.
Cited by
-
Asymmetric effects of activating and inactivating cortical interneurons.Elife. 2016 Oct 10;5:e18383. doi: 10.7554/eLife.18383. Elife. 2016. PMID: 27719761 Free PMC article.
-
Retinal direction of motion is reliably transmitted to visual cortex through highly selective thalamocortical connections.Curr Biol. 2025 Jan 6;35(1):217-223.e4. doi: 10.1016/j.cub.2024.11.013. Epub 2024 Dec 6. Curr Biol. 2025. PMID: 39644892 Free PMC article.
-
On the Diverse Functions of Electrical Synapses.Front Cell Neurosci. 2022 Jun 9;16:910015. doi: 10.3389/fncel.2022.910015. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35755782 Free PMC article. Review.
-
CaMKIIα-Positive Interneurons Identified via a microRNA-Based Viral Gene Targeting Strategy.J Neurosci. 2020 Dec 9;40(50):9576-9588. doi: 10.1523/JNEUROSCI.2570-19.2020. Epub 2020 Nov 6. J Neurosci. 2020. PMID: 33158963 Free PMC article.
-
Somatostatin-expressing neurons in cortical networks.Nat Rev Neurosci. 2016 Jul;17(7):401-9. doi: 10.1038/nrn.2016.53. Epub 2016 May 26. Nat Rev Neurosci. 2016. PMID: 27225074 Free PMC article. Review.
References
-
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol 82: 1295–1302, 1999a. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

