Design, synthesis and antibacterial potential of 5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-1-substituted-4,5-dihydropyrazoles
- PMID: 25972742
- PMCID: PMC4421084
- DOI: 10.1016/j.jsps.2014.07.009
Design, synthesis and antibacterial potential of 5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-1-substituted-4,5-dihydropyrazoles
Abstract
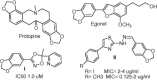
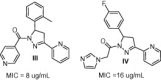
A series of 5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-1-substituted-4,5-dihydropyrazole derivatives 4a-e and 6a-g have been synthesized and spectrally characterized. The antibacterial activity of the novel candidates has been screened using the agar diffusion test. These compounds were endowed with high antibacterial activity against different Gram +ve and Gram -ve bacteria when compared with standard antibacterial drugs. In the light of zone of inhibition and MIC results, Sarcina and Staphylococcus aureus are the most sensitive bacteria where pyrrolidinomethanone derivative 4e showed MICs at 80 and 110 nM, respectively. While hydroxypiperidinoethanone derivative 6c showed MIC at 90 nM for Sarcina.
Keywords: 1,3-Benzodioxole; 2-Pyrazoline; Antibacterial.
Figures


References
-
- Aboul-Enein M.N., El-Azzouny A.A., Attia M.I., Maklad Y.A., Amin K.M., Abdel-Rehim M., El-Behairy M.F. Design and synthesis of novel stiripentol analogues as potential anticonvulsants. Eur. J. Med. Chem. 2012;47:360–369. - PubMed
-
- Aghatabay N.M., Mahmiani Y., Cevik H., Dulger B. Synthesis, Raman, FT-IR, NMR spectroscopic data and antimicrobial activity of mixed aza-oxo-thia macrocyclic compounds. Eur. J. Med. Chem. 2009;44:365–372. - PubMed
-
- Akbas E., Berber I. Antibacterial and antifungal activities of new pyrazolo[3,4-d]pyridazin derivatives. Eur. J. Med. Chem. 2005;40:401–405. - PubMed
-
- Al-Abbas M.J.A. Antimicrobial susceptibility of Enterococcus faecalis and a novel Planomicrobium isolate of bacteremia. Int. J. Med. Med. Sci. 2012;4:19–27.
-
- Baldwin J.J., Lumma W.C., Lundell G.F., Ponticello G.S., Raab A.W., Engelhardt E.L., Hirschmann R., Sweet C.S., Scriabine A. Symbiotic approach to drug design: antihypertensive beta-adrenergic blocking agents. J. Med. Chem. 1979;22:1284–1290. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

