Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance
- PMID: 25972811
- PMCID: PMC4412121
- DOI: 10.3389/fphys.2015.00116
Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance
Abstract
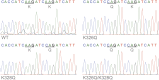
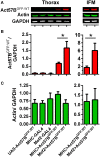
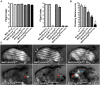
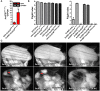
In striated muscle tropomyosin (Tm) extends along the length of F-actin-containing thin filaments. Its location governs access of myosin binding sites on actin and, hence, force production. Intermolecular electrostatic associations are believed to mediate critical interactions between the proteins. For example, actin residues K326, K328, and R147 were predicted to establish contacts with E181 of Tm. Moreover, K328 also potentially forms direct interactions with E286 of myosin when the motor is strongly bound. Recently, LC-MS/MS analysis of the cardiac acetyl-lysine proteome revealed K326 and K328 of actin were acetylated, a post-translational modification (PTM) that masks the residues' inherent positive charges. Here, we tested the hypothesis that by removing the vital actin charges at residues 326 and 328, the PTM would perturb Tm positioning and/or strong myosin binding as manifested by altered skeletal muscle function and structure in the Drosophila melanogaster model system. Transgenic flies were created that permit tissue-specific expression of K326Q, K328Q, or K326Q/K328Q acetyl-mimetic actin and of wild-type actin via the UAS-GAL4 bipartite expression system. Compared to wild-type actin, muscle-restricted expression of mutant actin had a dose-dependent effect on flight ability. Moreover, excessive K328Q and K326Q/K328Q actin overexpression induced indirect flight muscle degeneration, a phenotype consistent with hypercontraction observed in other Drosophila myofibrillar mutants. Based on F-actin-Tm and F-actin-Tm-myosin models and on our physiological data, we conclude that acetylating K326 and K328 of actin alters electrostatic associations with Tm and/or myosin and thereby augments contractile properties. Our findings highlight the utility of Drosophila as a model that permits efficient targeted design and assessment of molecular and tissue-specific responses to muscle protein modifications, in vivo.
Keywords: acetylation; muscle contraction; myosin; post-translational modification; tropomyosin.
Figures



Similar articles
-
The actin 'A-triad's' role in contractile regulation in health and disease.J Physiol. 2020 Jul;598(14):2897-2908. doi: 10.1113/JP276741. Epub 2019 Mar 28. J Physiol. 2020. PMID: 30770548 Free PMC article. Review.
-
Lysine acetylation of F-actin decreases tropomyosin-based inhibition of actomyosin activity.J Biol Chem. 2020 Nov 13;295(46):15527-15539. doi: 10.1074/jbc.RA120.015277. Epub 2020 Sep 1. J Biol Chem. 2020. PMID: 32873710 Free PMC article.
-
Distortion of the Actin A-Triad Results in Contractile Disinhibition and Cardiomyopathy.Cell Rep. 2017 Sep 12;20(11):2612-2625. doi: 10.1016/j.celrep.2017.08.070. Cell Rep. 2017. PMID: 28903042 Free PMC article.
-
Regulation of contraction in striated muscle.Physiol Rev. 2000 Apr;80(2):853-924. doi: 10.1152/physrev.2000.80.2.853. Physiol Rev. 2000. PMID: 10747208 Review.
-
Drosophila ACT88F indirect flight muscle-specific actin is not N-terminally acetylated: a mutation in N-terminal processing affects actin function.J Mol Biol. 2000 Feb 4;295(5):1201-10. doi: 10.1006/jmbi.1999.3407. J Mol Biol. 2000. PMID: 10653697
Cited by
-
Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage.J Muscle Res Cell Motil. 2021 Jun;42(2):367-380. doi: 10.1007/s10974-021-09596-9. Epub 2021 Feb 17. J Muscle Res Cell Motil. 2021. PMID: 33595762 Free PMC article.
-
Structural and functional mechanisms of actin isoforms.FEBS J. 2025 Feb;292(3):468-482. doi: 10.1111/febs.17153. Epub 2024 May 23. FEBS J. 2025. PMID: 38779987 Free PMC article. Review.
-
Lysine acetylation of cytoskeletal proteins: Emergence of an actin code.J Cell Biol. 2020 Dec 7;219(12):e202006151. doi: 10.1083/jcb.202006151. J Cell Biol. 2020. PMID: 33044556 Free PMC article. Review.
-
Cofilin Loss in Drosophila Muscles Contributes to Muscle Weakness through Defective Sarcomerogenesis during Muscle Growth.Cell Rep. 2020 Jul 21;32(3):107893. doi: 10.1016/j.celrep.2020.107893. Cell Rep. 2020. PMID: 32697999 Free PMC article.
-
Posttranslational modifications of the cytoskeleton.Cytoskeleton (Hoboken). 2021 Apr;78(4):142-173. doi: 10.1002/cm.21679. Epub 2021 Jul 2. Cytoskeleton (Hoboken). 2021. PMID: 34152688 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

