Endogenous generation of hydrogen sulfide and its regulation in Shewanella oneidensis
- PMID: 25972854
- PMCID: PMC4412017
- DOI: 10.3389/fmicb.2015.00374
Endogenous generation of hydrogen sulfide and its regulation in Shewanella oneidensis
Abstract
Hydrogen sulfide (H2S) has been recognized as a physiological mediator with a variety of functions across all domains of life. In this study, mechanisms of endogenous H2S generation in Shewanella oneidensis were investigated. As a research model with highly diverse anaerobic respiratory pathways, the microorganism is able to produce H2S by respiring on a variety of sulfur-containing compounds with SirACD and PsrABC enzymatic complexes, as well as through cysteine degradation with three enzymes, MdeA, SO_1095, and SseA. We showed that the SirACD and PsrABC complexes, which are predominantly, if not exclusively, responsible for H2S generation via respiration of sulfur species, do not interplay with each other. Strikingly, a screen for regulators controlling endogenous H2S generation by transposon mutagenesis identified global regulator Crp to be essential to all H2S-generating processes. In contrast, Fnr and Arc, two other global regulators that have a role in respiration, are dispensable in regulating H2S generation via respiration of sulfur species. Interestingly, Arc is involved in the H2S generation through cysteine degradation by repressing expression of the mdeA gene. We further showed that expression of the sirA and psrABC operons is subjected to direct regulation of Crp, but the mechanisms underlying the requirement of Crp for H2S generation through cysteine degradation remain elusive.
Keywords: Crp; H2S; Shewanella; endogenous generation; regulation.
Figures


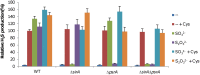


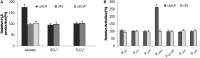
Similar articles
-
[Endogenous production and physiological functions of hydrogen sulfide in facultative anaerobic bacteria].Wei Sheng Wu Xue Bao. 2017 Feb 4;57(2):170-8. Wei Sheng Wu Xue Bao. 2017. PMID: 29750479 Review. Chinese.
-
A Matter of Timing: Contrasting Effects of Hydrogen Sulfide on Oxidative Stress Response in Shewanella oneidensis.J Bacteriol. 2015 Nov;197(22):3563-72. doi: 10.1128/JB.00603-15. Epub 2015 Aug 31. J Bacteriol. 2015. PMID: 26324455 Free PMC article.
-
Generation of hydrogen sulfide from sulfur assimilation in Escherichia coli.J Gen Appl Microbiol. 2019 Dec 19;65(5):234-239. doi: 10.2323/jgam.2018.11.001. Epub 2019 Mar 15. J Gen Appl Microbiol. 2019. PMID: 30880290
-
Mechanism of H2S Oxidation by the Dissimilatory Perchlorate-Reducing Microorganism Azospira suillum PS.mBio. 2017 Feb 21;8(1):e02023-16. doi: 10.1128/mBio.02023-16. mBio. 2017. PMID: 28223460 Free PMC article.
-
Central Role of Adenosine 5'-Phosphosulfate Reductase in the Control of Plant Hydrogen Sulfide Metabolism.Front Plant Sci. 2018 Sep 24;9:1404. doi: 10.3389/fpls.2018.01404. eCollection 2018. Front Plant Sci. 2018. PMID: 30319669 Free PMC article. Review.
Cited by
-
Shewanella oneidensis arcA Mutation Impairs Aerobic Growth Mainly by Compromising Translation.Life (Basel). 2021 Sep 6;11(9):926. doi: 10.3390/life11090926. Life (Basel). 2021. PMID: 34575075 Free PMC article.
-
NapB in excess inhibits growth of Shewanella oneidensis by dissipating electrons of the quinol pool.Sci Rep. 2016 Nov 18;6:37456. doi: 10.1038/srep37456. Sci Rep. 2016. PMID: 27857202 Free PMC article.
-
Cbl upregulates cysH for hydrogen sulfide production in Aeromonas veronii.PeerJ. 2021 Sep 9;9:e12058. doi: 10.7717/peerj.12058. eCollection 2021. PeerJ. 2021. PMID: 34589297 Free PMC article.
-
"On demand" redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor.Chem Sci. 2017 Jul 1;8(7):4967-4972. doi: 10.1039/c7sc00873b. Epub 2017 Apr 27. Chem Sci. 2017. PMID: 28959420 Free PMC article.
-
A novel bacterial sulfite dehydrogenase that requires three c-type cytochromes for electron transfer.Appl Environ Microbiol. 2023 Oct 31;89(10):e0110823. doi: 10.1128/aem.01108-23. Epub 2023 Sep 21. Appl Environ Microbiol. 2023. PMID: 37732808 Free PMC article.
References
-
- Charania M. A., Brockman K. L., Zhang Y., Banerjee A., Pinchuk G. E., Fredrickson J. K., et al. . (2009). Involvement of a membrane-bound class III adenylate cyclase in regulation of anaerobic respiration in Shewanella oneidensis MR-1. J. Bacteriol. 191, 4298–4306. 10.1128/JB.01829-08 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

