Host cell type-dependent translocation and PhoP-mediated positive regulation of the effector SseK1 of Salmonella enterica
- PMID: 25972862
- PMCID: PMC4413795
- DOI: 10.3389/fmicb.2015.00396
Host cell type-dependent translocation and PhoP-mediated positive regulation of the effector SseK1 of Salmonella enterica
Abstract
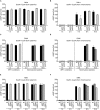
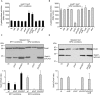
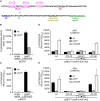
Salmonella enterica expresses two virulence-related type III secretion systems (T3SSs) encoded in Salmonella pathogenicity island 1 (SPI1) and SPI2, respectively. SseK1 is a poorly characterized substrate of the SPI2-encoded T3SS. Here, we show that this effector is essential to get full virulence both in oral and intraperitoneal mice infections, in spite of not having a role in invasion or intracellular proliferation in cultured mammalian cells. In vitro, expression of sseK1 was higher in media mimicking intracellular conditions, when SPI2 was induced, but it was also significant under SPI1 inducing conditions. A detailed analysis of translocation of SseK1 into host cells unveiled that it was a substrate of both, T3SS1 and T3SS2, although with different patterns and kinetics depending on the specific host cell type (epithelial, macrophages, or fibroblasts). The regulation of the expression of sseK1 was examined using lacZ and bioluminescent lux fusions. The two-component system PhoQ/PhoP is a positive regulator of this gene. A combination of sequence analysis, directed mutagenesis and electrophoretic mobility shift assays showed that phosphorylated PhoP binds directly to the promoter region of sseK1 and revealed a PhoP binding site located upstream of the predicted -35 hexamer of this promoter.
Keywords: PhoQ/PhoP two-component system; Salmonella; SseK1; bioluminescence; epithelial cells; fibroblasts; macrophages; type III secretion.
Figures
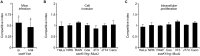


Similar articles
-
SseK1 and SseK2 are novel translocated proteins of Salmonella enterica serovar typhimurium.Infect Immun. 2004 Sep;72(9):5115-25. doi: 10.1128/IAI.72.9.5115-5125.2004. Infect Immun. 2004. PMID: 15322005 Free PMC article.
-
Patterns of expression and translocation of the ubiquitin ligase SlrP in Salmonella enterica serovar Typhimurium.J Bacteriol. 2014 Nov;196(22):3912-22. doi: 10.1128/JB.02158-14. Epub 2014 Sep 2. J Bacteriol. 2014. PMID: 25182488 Free PMC article.
-
PhoP-Mediated Repression of the SPI1 Type 3 Secretion System in Salmonella enterica Serovar Typhimurium.J Bacteriol. 2019 Jul 24;201(16):e00264-19. doi: 10.1128/JB.00264-19. Print 2019 Aug 15. J Bacteriol. 2019. PMID: 31182495 Free PMC article.
-
PhoP/PhoQ: macrophage-specific modulators of Salmonella virulence?Mol Microbiol. 1991 Sep;5(9):2073-8. doi: 10.1111/j.1365-2958.1991.tb02135.x. Mol Microbiol. 1991. PMID: 1766380 Review.
-
Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics resistance of Salmonella enterica.Berl Munch Tierarztl Wochenschr. 2007 Jul-Aug;120(7-8):317-27. Berl Munch Tierarztl Wochenschr. 2007. PMID: 17715824 Review.
Cited by
-
Arg-GlcNAcylation on TRADD by NleB and SseK1 Is Crucial for Bacterial Pathogenesis.Front Cell Dev Biol. 2020 Jul 17;8:641. doi: 10.3389/fcell.2020.00641. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32766249 Free PMC article.
-
Novel Template Plasmids pCyaA'-Kan and pCyaA'-Cam for Generation of Unmarked Chromosomal cyaA' Translational Fusion to T3SS Effectors in Salmonella.Microorganisms. 2021 Feb 25;9(3):475. doi: 10.3390/microorganisms9030475. Microorganisms. 2021. PMID: 33668764 Free PMC article.
-
Speaking the host language: how Salmonella effector proteins manipulate the host.Microbiology (Reading). 2023 Jun;169(6):001342. doi: 10.1099/mic.0.001342. Microbiology (Reading). 2023. PMID: 37279149 Free PMC article. Review.
-
Tubulin Folding Cofactor TBCB is a Target of the Salmonella Effector Protein SseK1.Int J Mol Sci. 2020 Apr 30;21(9):3193. doi: 10.3390/ijms21093193. Int J Mol Sci. 2020. PMID: 32366039 Free PMC article.
-
Salmonella T3SS effector SseK1 arginine-glycosylates the two-component response regulator OmpR to alter bile salt resistance.Sci Rep. 2023 Jun 3;13(1):9018. doi: 10.1038/s41598-023-36057-9. Sci Rep. 2023. PMID: 37270573 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

