Therapeutic potential and challenges of natural killer cells in treatment of solid tumors
- PMID: 25972872
- PMCID: PMC4413815
- DOI: 10.3389/fimmu.2015.00202
Therapeutic potential and challenges of natural killer cells in treatment of solid tumors
Abstract
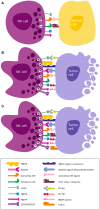
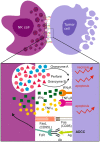
Natural killer (NK) cells are innate lymphoid cells that hold tremendous potential for effective immunotherapy for a broad range of cancers. Due to the mode of NK cell killing, requiring one-to-one target engagement and site-directed release of cytolytic granules, the therapeutic potential of NK cells has been most extensively explored in hematological malignancies. However, their ability to precisely kill antibody coated cells, cancer stem cells, and genotoxically altered cells, while maintaining tolerance to healthy cells makes them appealing therapeutic effectors for all cancer forms, including metastases. Due to their release of pro-inflammatory cytokines, NK cells may potently reverse the anti-inflammatory tumor microenvironment (TME) and augment adaptive immune responses by promoting differentiation, activation, and/or recruitment of accessory immune cells to sites of malignancy. Nevertheless, integrated and coordinated mechanisms of subversion of NK cell activity against the tumor and its microenvironment exist. Although our understanding of the receptor ligand interactions that regulate NK cell functionality has evolved remarkably, the diversity of ligands and receptors is complex, as is their mechanistic foundations in regulating NK cell function. In this article, we review the literature and highlight how the TME manipulates the NK cell phenotypes, genotypes, and tropism to evade tumor recognition and elimination. We discuss counter strategies that may be adopted to augment the efficacy of NK cell anti-tumor surveillance, the clinical trials that have been undertaken so far in solid malignancies, critically weighing the challenges and opportunities with this approach.
Keywords: KIR-HLA interactions; NK-cell subsets; cancer stem cells; clinical application; tumor microenvironment.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

