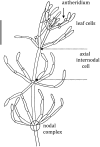
Salt tolerance at single cell level in giant-celled Characeae
- PMID: 25972875
- PMCID: PMC4412000
- DOI: 10.3389/fpls.2015.00226
Salt tolerance at single cell level in giant-celled Characeae
Abstract
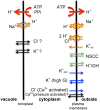
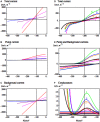
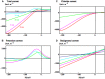
Characean plants provide an excellent experimental system for electrophysiology and physiology due to: (i) very large cell size, (ii) position on phylogenetic tree near the origin of land plants and (iii) continuous spectrum from very salt sensitive to very salt tolerant species. A range of experimental techniques is described, some unique to characean plants. Application of these methods provided electrical characteristics of membrane transporters, which dominate the membrane conductance under different outside conditions. With this considerable background knowledge the electrophysiology of salt sensitive and salt tolerant genera can be compared under salt and/or osmotic stress. Both salt tolerant and salt sensitive Characeae show a rise in membrane conductance and simultaneous increase in Na(+) influx upon exposure to saline medium. Salt tolerant Chara longifolia and Lamprothamnium sp. exhibit proton pump stimulation upon both turgor decrease and salinity increase, allowing the membrane PD to remain negative. The turgor is regulated through the inward K(+) rectifier and 2H(+)/Cl(-) symporter. Lamprothamnium plants can survive in hypersaline media up to twice seawater strength and withstand large sudden changes in salinity. Salt sensitive C. australis succumbs to 50-100 mM NaCl in few days. Cells exhibit no pump stimulation upon turgor decrease and at best transient pump stimulation upon salinity increase. Turgor is not regulated. The membrane PD exhibits characteristic noise upon exposure to salinity. Depolarization of membrane PD to excitation threshold sets off trains of action potentials, leading to further loses of K(+) and Cl(-). In final stages of salt damage the H(+)/OH(-) channels are thought to become the dominant transporter, dissipating the proton gradient and bringing the cell PD close to 0. The differences in transporter electrophysiology and their synergy under osmotic and/or saline stress in salt sensitive and salt tolerant characean cells are discussed in detail.
Keywords: Characeae; H+/OH- channels; action potentials; current-voltage characteristics; electrophysiology; non-selective cation channels; proton pump; salt tolerance.
Figures





References
-
- Al Khazaaly S., Beilby M. J. (2007). Modeling ion transporters at the time of hypertonic regulation Lamprothamnium succinctum (Characeae, Charophyceae). Charophytes 1 28–47.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

