Review of the experiences from the first childhood influenza vaccination programme with a live attenuated influenza vaccine in England and Scotland
- PMID: 25972905
- PMCID: PMC4423484
- DOI: 10.7573/dic.212280
Review of the experiences from the first childhood influenza vaccination programme with a live attenuated influenza vaccine in England and Scotland
Abstract
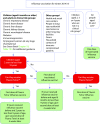
In 2012, the Joint Committee on Vaccination and Immunisation recommended that the National Immunisation Programme for influenza be extended to include healthy children/adolescents aged 2-17 years. In the UK, extension of this new immunisation programme began in 2013-2014 and targeted children aged 2 years and 3 years in primary care. Several implementation pilots were undertaken in primary schools across England, Scotland, Wales and Northern Ireland, as well as a single pilot in a secondary school in England. This article shares lessons learnt from experiences in England and Scotland to provide guidance for other countries considering the addition of childhood influenza vaccination into their national immunisation programmes. Recommendations are provided to help ensure effective preparation and management of new childhood influenza vaccination programmes in other countries. This article describes the processes utilised in England and Scotland for programme setup, workforce management, identification and care of contraindicated patients, collection of data on vaccine uptake, communication strategies, and education of parents and children.
Keywords: England; Scotland; children; influenza; pilot project; schools; vaccination; vaccine.
Figures
References
-
- Influenza: the Green Book, chapter 19. Available from: https://www.gov.uk/government/publications/influenza-the-green-book-chap.... Last accessed: 18 November 2014.
-
- Joint Committee on Vaccination and Immunisation Minutes of the meeting held on Friday 13 April 2012. Available from: http://media.dh.gov.uk/network/261/files/2012/05/JCVI-minutes-13-April-2.... Last accessed: 11 September 2014.
-
- Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, Biolek J, Kuhr J, Bujnowski T, Desgrandchamps D, Cheng SM, Skinner J, Gruber WC, Forrest BD. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870–9. doi: 10.1097/01.inf.0000237829.66310.85. - DOI - PubMed
-
- Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, Oymar K, Garcia ML, Krygier A, Costa H, Heininger U, Pregaldien JL, Cheng SM, Skinner J, Razmpour A, Saville M, Gruber WC, Forrest B. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25:860–9. doi: 10.1097/01.inf.0000237797.14283.cf. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

