Phosphorylation on TRPV4 Serine 824 Regulates Interaction with STIM1
- PMID: 25972993
- PMCID: PMC4412957
- DOI: 10.2174/1874091X01509010024
Phosphorylation on TRPV4 Serine 824 Regulates Interaction with STIM1
Abstract
The TRPV4 cation channel, a member of the TRP vanilloid subfamily, is expressed in a broad range of tissues where it participates in the generation of a Ca2+ signal and/or depolarization of membrane potential. Here, we identified stromal interaction molecule 1 precursor (STIM1) as an auxiliary protein of this epithelial Ca2+channel using confocal microscopy analysis and GST pull-down assay. The STIM1 protein associates specifically with the C-terminal tail of TRPV4 to form a complex. In previous reports, we demonstrated that the serine824 residue of TRPV4 is one of the target phosphorylation sites of serum/glucocorticoid regulated kinase 1 (SGK1). In this report we further identified the role of serine 824 phosphorylation. The TRPV4 mutant S824D (not S824A) exhibited a diminished capacity to bind STIM1. Using GST pull-down and co-immunoprecipitation assays, we demonstrated that STIM1 is part of the TRPV4 protein complex. Our observations clearly suggest that the formation of a complex between TRPV4 and STIM1 and its plasma membrane localization are regulated through phosphorylation of serine824 of TRPV4, and that the STIM1-TRPV4 complex plays crucial roles in routing TRPV4 to the plasma membrane from the endoplasmic reticulum and in maintaining its function.
Keywords: Channel; STIM1; TRPV4; phosphorylation; protein-protein interaction.
Figures
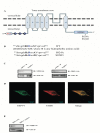

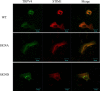


Similar articles
-
Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F-actin than with microtubules to expand the cell surface area.Cell Signal. 2012 Mar;24(3):641-51. doi: 10.1016/j.cellsig.2011.11.002. Epub 2011 Nov 10. Cell Signal. 2012. PMID: 22101010
-
The negative feedback regulation of TRPV4 Ca2+ ion channel function by its C-terminal cytoplasmic domain.Cell Signal. 2012 Oct;24(10):1918-22. doi: 10.1016/j.cellsig.2012.06.008. Epub 2012 Jun 23. Cell Signal. 2012. PMID: 22735813 Review.
-
Filamin A Modulates Store-Operated Ca2+ Entry by Regulating STIM1 (Stromal Interaction Molecule 1)-Orai1 Association in Human Platelets.Arterioscler Thromb Vasc Biol. 2018 Feb;38(2):386-397. doi: 10.1161/ATVBAHA.117.310139. Epub 2017 Dec 28. Arterioscler Thromb Vasc Biol. 2018. PMID: 29284605
-
Fast endocytic recycling determines TRPC1-STIM1 clustering in ER-PM junctions and plasma membrane function of the channel.Biochim Biophys Acta. 2015 Oct;1853(10 Pt A):2709-21. doi: 10.1016/j.bbamcr.2015.07.019. Epub 2015 Jul 30. Biochim Biophys Acta. 2015. PMID: 26232624
-
Regulation of Orai1/STIM1 by the kinases SGK1 and AMPK.Cell Calcium. 2012 Nov;52(5):347-54. doi: 10.1016/j.ceca.2012.05.005. Epub 2012 Jun 7. Cell Calcium. 2012. PMID: 22682960 Review.
Cited by
-
Structure of the human TRPM4 ion channel in a lipid nanodisc.Science. 2018 Jan 12;359(6372):228-232. doi: 10.1126/science.aar4510. Epub 2017 Dec 7. Science. 2018. PMID: 29217581 Free PMC article.
-
TRPing to the Point of Clarity: Understanding the Function of the Complex TRPV4 Ion Channel.Cells. 2021 Jan 15;10(1):165. doi: 10.3390/cells10010165. Cells. 2021. PMID: 33467654 Free PMC article. Review.
-
Consideration of Kinase Inhibitors for the Treatment of Hydrocephalus.Int J Mol Sci. 2023 Apr 3;24(7):6673. doi: 10.3390/ijms24076673. Int J Mol Sci. 2023. PMID: 37047646 Free PMC article. Review.
-
TRPV4 controls circadian and pathological ocular hypertension.J Physiol. 2025 Jul;603(14):4091-4111. doi: 10.1113/JP288706. Epub 2025 Jul 10. J Physiol. 2025. PMID: 40638660 Free PMC article.
-
Phosphorylation of distal C-terminal residues promotes TRPV4 channel activation in response to arachidonic acid.J Biol Chem. 2025 Mar;301(3):108260. doi: 10.1016/j.jbc.2025.108260. Epub 2025 Feb 3. J Biol Chem. 2025. PMID: 39909371 Free PMC article.
References
-
- Nishimura G, Lausch E, Savarirayan R, Shiba M, Spranger J, Zabel B, Ikegawa S, Superti-Furga A, Unger S. TRPV4-associated skeletal dysplasias. Am. J. Med. Genet. C. Semin. Med. Genet. 2012;160C(3):190–204. - PubMed
-
- Lang F, Shumilina E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J. 2013;27:3–12. - PubMed
-
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis RX Xu. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2 J. Pharmacol. Exp. Ther. 2008;326:443–45. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
