Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins
- PMID: 25973164
- PMCID: PMC4429923
- DOI: 10.1186/s13229-015-0004-5
Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins
Abstract
Background: Autism spectrum disorders (ASDs) are more prevalent in males, suggesting a multiple threshold liability model in which females are, on average, protected by sex-differential mechanisms. Under this model, autistic females are predicted to carry a more penetrant risk variant load than males and to share this greater genetic liability with their siblings. However, reported ASD recurrence rates have not demonstrated significantly increased risk to siblings of affected girls. Here, we characterize recurrence patterns in multiplex families from the Autism Genetics Resource Exchange (AGRE) to determine if risk in these families follows a female protective model.
Methods: We assess recurrence rates and quantitative traits in full siblings from 1,120 multiplex nuclear families and concordance rates in 305 twin pairs from AGRE. We consider the first two affected children per family, and one randomly selected autistic twin per pair, as probands. We then compare recurrence rates and phenotypes between males and females and between twin pairs or families with at least one female proband (female-containing (FC)) versus those with only male probands (male-only (MO)).
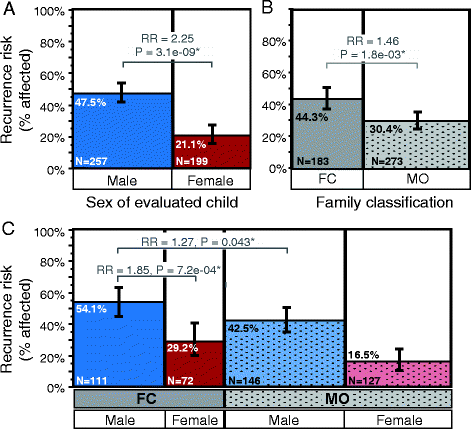
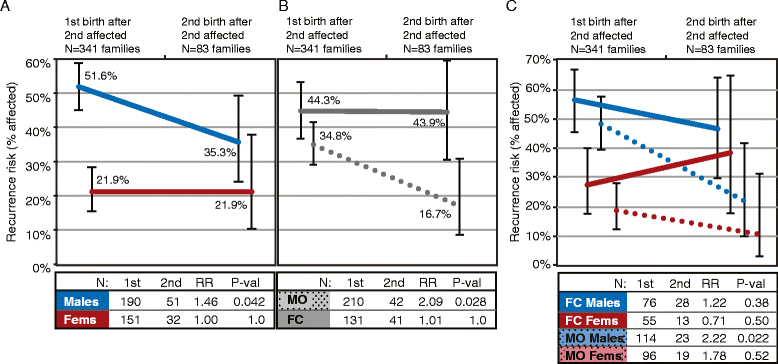
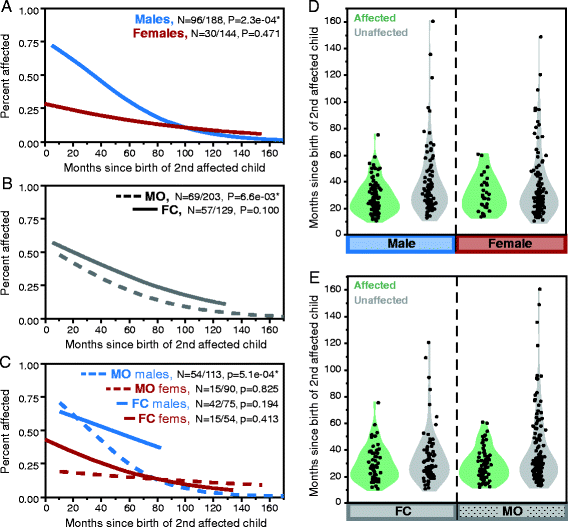
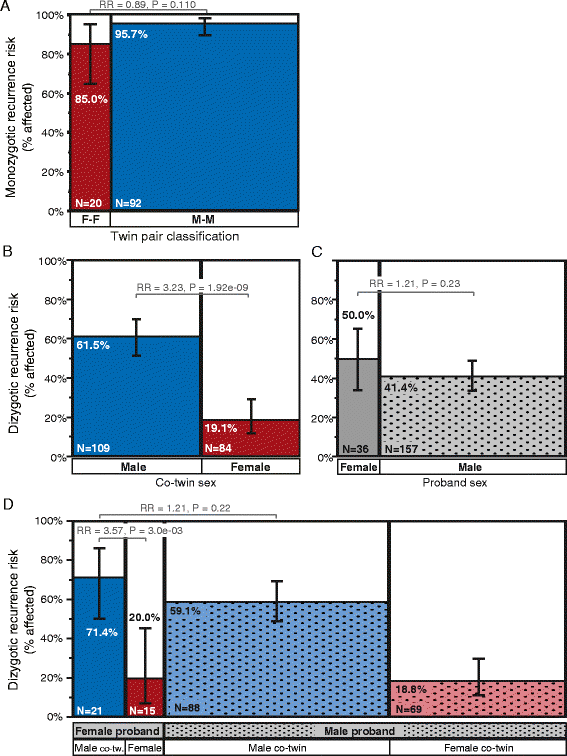
Results: Among children born after two probands, we observe significantly higher recurrence in males (47.5%) than in females (21.1%; relative risk, RR = 2.25; adjusted P = 6.22e-08) and in siblings of female (44.3%) versus siblings of male probands (30.4%; RR = 1.46; adj. P = 0.036). This sex-differential recurrence is also robust in dizygotic twin pairs (males = 61.5%, females = 19.1%; RR = 3.23; adj. P = 7.66e-09). Additionally, we find a significant negative relationship between interbirth interval and ASD recurrence that is driven by children in MO families.
Conclusions: By classifying families as MO or FC using two probands instead of one, we observe significant recurrence rate differences between families harboring sex-differential familial liability. However, a significant sex difference in risk to children within FC families suggests that female protective mechanisms are still operative in families carrying high genetic risk loads. Furthermore, the male-specific relationship between shorter interbirth intervals and increased ASD risk is consistent with a potentially greater contribution from environmental factors in males versus higher genetic risk in affected females and their families. Understanding the mechanisms driving these sex-differential risk profiles will be useful for treatment development and prevention.
Keywords: AGRE; Female protective model; Interbirth interval; Multiplex families; Recurrence risk; Sex differences.
Figures
References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Association; 2013.
-
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators CfDCaP. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1–21. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

