Nanog down-regulates the Wnt signaling pathway via β-catenin phosphorylation during epidermal stem cell proliferation and differentiation
- PMID: 25973172
- PMCID: PMC4429823
- DOI: 10.1186/2045-3701-5-5
Nanog down-regulates the Wnt signaling pathway via β-catenin phosphorylation during epidermal stem cell proliferation and differentiation
Abstract
Background: Skin tissue homeostasis is maintained by a balance between the proliferation and differentiation of epidermal stem cells (EpSCs). EpSC proliferation and differentiation are complex processes regulated by many factors and signaling pathways. This study aimed to explore the connection between the Nanog and the Wnt/β-catenin pathway in the proliferation and differentiation of EpSCs.
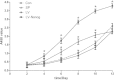
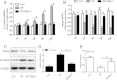
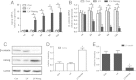
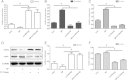
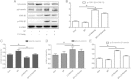
Results: Our results demonstrated that during the study period, EpSC underwent differentiation when incubated in the presence neuropeptide substance P (SP), there was an opposing expression trend of Nanog and β-catenin after SP treatment, which could be antagonized by the Wnt antagonist, Dkk-1. The transduced EpSCs had a greater proliferative ability than the SP treatment group and they did not undergo differentiation upon SP treatment. More important, β-catenin expression was down-regulated but phosphorylated β-catenin expression and phosphorylated GSK-3β expression was up-regulated upon Nanog overexpression.
Conclusions: These results strongly suggest that Nanog plays an important role in maintaining the proliferation and differentiation homeostasis of EpSCs by promoting β-catenin phosphorylation via GSK-3β to inhibit the activity of the Wnt/β-catenin signaling pathway. This is important for precise regulation of proliferation and differentiation of EpSC in the application of tissue engineering.
Keywords: Differentiation; Epidermal stem cells; Nanog; Proliferation; Wnt/β-catenin.
Figures


References
-
- Chai L, Cao C, Bi S, Dai X, Gan L, Guo R, et al. Small Rho GTPase Rac1 determines human epidermal stem cell fate in vitro. Int J Mol Med. 2010;25(5):723–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

