Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis
- PMID: 25973226
- PMCID: PMC4419320
- DOI: 10.3978/j.issn.2072-1439.2015.03.10
Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis
Abstract
Background: Linezolid containing regimens have been proposed as potentially valuable alternatives for the treatment of patients with multidrug-resistant tuberculosis (MDR-TB) or extensively drug-resistant TB (XDR-TB).
Methods: A systematic review and meta-analysis was conducted to assess the efficacy, safety and tolerability of linezolid for drug-resistant TB (DR-TB) treatment. We searched the Cochrane Controlled Trial Registry, PubMed, Embase, Science Citation Index Expanded (SCI) and China National Knowledge Infrastructure (CNKI), database up to May 2014 to identify studies providing data of the use of linezolid for the treatment of DR-TB.
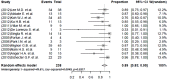
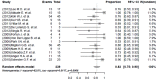
Results: The search yielded 15 studies (367 patients) including one randomized controlled trial (RCT), covering 239 patients who could be evaluated for effectiveness; 83% [95% confidence interval (CI), 75-90%; I(2)=62.8%] had a favorable outcome, defined as either cure or treatment completion. The pooled rate of culture conversion was 89% (95% CI, 83-95%; I(2)=49.6%). Between the group receiving daily linezolid doses of ≤600 or >600 mg, the mortality was considerably lower in patients treated with less than 600 mg/day (P value <0.001). Of 367 patients for whom data on safety was available, peripheral neuropathy (31%, 95% CI, 19-42%; I(2)=81.7%) and anemia (25%, 95% CI, 15-34%; I(2)=76.6%) were the main adverse effects. Patients receiving less than 600 mg/day were more likely to experience nervous system adverse events (P value <0.01).
Conclusions: The available evidence suggests that linezolid could be considered as a promising option as treatment of MDR/XDR TB. Randomized trials are warranted to define the dose and frequency of administration.
Keywords: Multidrug-resistant tuberculosis (MDR-TB); extensively drug-resistant TB (XDR-TB); linezolid; meta-analysis; treatment.
Figures




References
-
- World Health Organization. Global tuberculosis report 2013. World Health Organization, 2013.
-
- Dye C, Glaziou P, Floyd K, et al. Prospects for tuberculosis elimination. Annu Rev Public Health 2013;34:271-86. - PubMed
-
- Yang C, Lei H, Wang D, et al. In vitro activity of linezolid against clinical isolates of Mycobacterium tuberculosis, including multidrug-resistant and extensively drug-resistant strains from Beijing, China. Jpn J Infect Dis 2012;65:240-2. - PubMed
LinkOut - more resources
Full Text Sources
Medical
