Semenogelin I promotes prostate cancer cell growth via functioning as an androgen receptor coactivator and protecting against zinc cytotoxicity
- PMID: 25973311
- PMCID: PMC4396034
Semenogelin I promotes prostate cancer cell growth via functioning as an androgen receptor coactivator and protecting against zinc cytotoxicity
Abstract
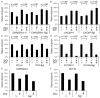
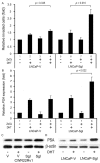
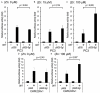
A seminal plasma protein, semenogelin I (SgI), contributes to sperm clotting, upon binding to Zn(2+), and can be proteolyzed by prostate-specific antigen (PSA), resulting in release of the trapped spermatozoa after ejaculation. In contrast, the role of SgI in the development and progression of any types of malignancies remains largely unknown. We previously demonstrated that SgI was overexpressed in prostate cancer tissues and its expression was enhanced by zinc treatment in LNCaP cells. In the current study, using cell lines stably expressing SgI, we investigated its biological functions, in conjunction with zinc, androgen, and androgen receptor (AR), in prostate cancer. Zinc, without SgI, inhibited cell growth of both AR-positive and AR-negative lines. Co-expression of SgI prevented zinc inhibiting dihydrotestosterone-mediated proliferation of AR-positive cells, whereas SgI and/or dihydrotestosterone showed marginal effects in AR-negative cells. Similar effects of SgI overexpression in LNCaP on dihydrotestosterone-induced cell invasion, such as its significant enhancement with zinc, were seen. Overexpression of SgI in LNCaP and CWR22Rv1 cells also augmented dihydrotestosterone-mediated PSA expression (mRNA, protein) in the presence of zinc. However, culture in the conditioned medium containing secreted forms of SgI failed to significantly increase cell viability with or without zinc. In luciferase reporter gene assays, SgI showed even slight inhibitory effects (8% and 15% decreases in PC3 and CWR22Rv1, respectively) at 0 μM zinc and significant stimulatory effects (2.1- and 3.2-fold) at 100 μM zinc on dihydrotestosterone-enhanced AR transactivation. Co-immunoprecipitation then demonstrated dihydrotestosterone-induced physical interactions between AR and SgI. These results suggest that intracellular SgI, together with zinc, functions as an AR coactivator and thereby promotes androgen-mediated prostate cancer progression.
Keywords: Androgen receptor; prostate cancer; prostate-specific antigen; semenogelin; zinc.
Figures


Similar articles
-
The interaction between androgen receptor and semenogelin I: a synthetic LxxLL peptide antagonist inhibits the growth of prostate cancer cells.Br J Cancer. 2018 Feb 6;118(3):416-420. doi: 10.1038/bjc.2017.404. Epub 2017 Nov 14. Br J Cancer. 2018. PMID: 29136406 Free PMC article.
-
Tumor necrosis factor-alpha represses androgen sensitivity in the LNCaP prostate cancer cell line.J Urol. 2000 Sep;164(3 Pt 1):800-5. doi: 10.1097/00005392-200009010-00053. J Urol. 2000. PMID: 10953159
-
Corepressive function of nuclear receptor coactivator 2 in androgen receptor of prostate cancer cells treated with antiandrogen.BMC Cancer. 2016 May 25;16:332. doi: 10.1186/s12885-016-2378-y. BMC Cancer. 2016. PMID: 27225190 Free PMC article.
-
Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models.Prostate. 2003 Mar 1;54(4):249-57. doi: 10.1002/pros.10199. Prostate. 2003. PMID: 12539223
-
Androgen receptor and TGFbeta1/Smad signaling are mutually inhibitory in prostate cancer.Eur Urol. 2005 Dec;48(6):1051-8. doi: 10.1016/j.eururo.2005.09.006. Epub 2005 Oct 14. Eur Urol. 2005. PMID: 16257107
Cited by
-
The interaction between androgen receptor and semenogelin I: a synthetic LxxLL peptide antagonist inhibits the growth of prostate cancer cells.Br J Cancer. 2018 Feb 6;118(3):416-420. doi: 10.1038/bjc.2017.404. Epub 2017 Nov 14. Br J Cancer. 2018. PMID: 29136406 Free PMC article.
-
Impact of Vasectomy on the Development and Progression of Prostate Cancer: Preclinical Evidence.Cancers (Basel). 2020 Aug 15;12(8):2295. doi: 10.3390/cancers12082295. Cancers (Basel). 2020. PMID: 32824199 Free PMC article.
-
SEMG1/2 augment energy metabolism of tumor cells.Cell Death Dis. 2020 Dec 11;11(12):1047. doi: 10.1038/s41419-020-03251-w. Cell Death Dis. 2020. PMID: 33311447 Free PMC article.
-
Surface protein profiling of prostate-derived extracellular vesicles by mass spectrometry and proximity assays.Commun Biol. 2022 Dec 22;5(1):1402. doi: 10.1038/s42003-022-04349-x. Commun Biol. 2022. PMID: 36550367 Free PMC article.
-
Emerging roles of cancer-testis antigenes, semenogelin 1 and 2, in neoplastic cells.Cell Death Discov. 2021 May 8;7(1):97. doi: 10.1038/s41420-021-00482-4. Cell Death Discov. 2021. PMID: 33966049 Free PMC article. Review.
References
-
- Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:265–271. - PubMed
-
- Rahman M, Miyamoto H, Chang C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin Cancer Res. 2004;10:2208–2219. - PubMed
-
- Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer. 2007;120:719–733. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
