INVESTIGATING THE EFFICACY OF CLINICAL TRIAL MONITORING STRATEGIES: Design and Implementation of the Cluster Randomized START Monitoring Substudy
- PMID: 25973346
- PMCID: PMC4426264
- DOI: 10.1177/2168479014555912
INVESTIGATING THE EFFICACY OF CLINICAL TRIAL MONITORING STRATEGIES: Design and Implementation of the Cluster Randomized START Monitoring Substudy
Abstract
Background: Trial monitoring protects participant safety and study integrity. While monitors commonly go on-site to verify source data, there is little evidence that this practice is efficient or effective. An ongoing international HIV treatment trial (START) provides an opportunity to explore the usefulness of different monitoring approaches.
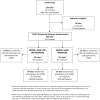
Methods: All START sites are centrally monitored and required to follow a local monitoring plan requiring specific quality assurance activities. Additionally, sites were randomized (1:1) to receive, or not receive, annual on-site monitoring. The study will determine if on-site monitoring increases the identification of major protocol deviations (eligibility or consent violations, improper study drug use, primary or serious event underreporting, data alteration or fraud).
Results: The START study completed enrollment in December 2013, with planned follow-up through December 2016. The monitoring study is ongoing at 196 sites in 34 countries. Results are expected when the START study concludes in December 2016.
Keywords: Data monitoring; centralized monitoring; clinical trials; on-site monitoring; quality assurance.
Conflict of interest statement
References
-
- Baigent C, Harrell F, Buyse M, et al. Ensuring trial validity by data quality assurance and diversification of monitoring methods. Clin Trials. 2008;5:49–55. - PubMed
-
- US Food and Drug Administration. [accessed 6 May 2014];Guidance for Industry Oversight of Clinical Investigations – A Risk-Based Approach to Monitoring. 2013 Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati....
-
- European Medicines Agency. [accessed 6 May 2014];Reflection paper on risk based quality management in clinical trials. 2011 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
-
- Usher RW. PhRMA BioResearch Monitoring Committee Perspective on Acceptable Approaches for Clinical Trial Monitoring. Drug Information Journal. 2010;44:477–483.
-
- Bakobaki J, Joffe N, Burdett S, et al. A systematic search for reports of site monitoring technique comparisons in clinical trials. Clin Trials. 2012;9:777–780. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials