Munc18c mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose stimulated insulin secretion in human pancreatic beta-cells
- PMID: 25973389
- PMCID: PMC4421095
- DOI: 10.1016/j.molmet.2015.02.004
Munc18c mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose stimulated insulin secretion in human pancreatic beta-cells
Abstract
Objective: Pancreatic beta-cells express three Munc18 isoforms. Much is known about the roles of Munc18a (pre-docked secretory granules-SGs) and Munc18b (newcomer SGs and SG-SG fusion) in insulin exocytosis. Although shown to influence glucose-stimulated insulin secretion (GSIS) in rodents the precise role of Munc18c in insulin SG exocytosis has not been elucidated. We here examined the role of Munc18c in human pancreatic beta-cells.
Methods: Munc18c-shRNA/RFP lenti-virus (versus control virus) was used to knock down the expression level of Munc18c in human islets or single beta-cells. Insulin secretion and granule exocytosis were measured by performing islets perifusion, single-cell patch clamp capacitance measurements and total internal reflection fluorescence microscopy (TIRFM).
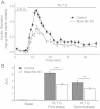
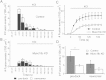
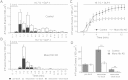
Results: Munc18c is most abundant in the cytosol of human beta-cells. Endogenous function of Munc18c was assessed by knocking down (KD) its islet expression by 70% employing lenti-shRNA virus. Munc18c-KD caused reduction in cognate syntaxin-4 islet expression but not in other exocytotic proteins, resulting in the reduction in GSIS in first- (by 42%) and second phases (by 35%). Single cell analyses of RFP-tagged Munc18c-KD beta-cells by patch clamp capacitance measurements showed inhibition in both readily-releasable pool (by 52%) and mobilization from the reserve pool (by 57%). TIRFM to assess single SG behavior showed that Munc18c-KD inhibition of first phase GSIS was attributed to reduction in exocytosis of pre-docked and newcomer SGs, and second phase inhibition attributed entirely to reduction in newcomer SG fusion (SGs that undergo minimal residence or docking time at the plasma membrane before fusion).
Conclusion: Munc18c is involved in the distinct molecular machineries that affect exocytosis of both predocked and newcomer SG pools that underlie Munc18c's role in first and second phases of GSIS, respectively.
Keywords: Ad, adenovirus; CmPatch, clamp capacitance measurements; EGFP, enhanced green fluorescent protein; Exocytosis; GLP-1, glucagon-like peptide-1; GSIS, glucose-stimulated insulin secretion; Human islets; KD, knock down; Munc18c; NPY, neuropeptide Y; Newcomer insulin granules; PM, plasma membrane; RRP, readily releasable pool; SG, secretory insulin-containing granule; SM, Sec1/Munc18-like protein; SNAP25/23, synaptosomal-associated protein of 25/23 kD; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; Syn, syntaxin; T2DM, type 2 diabetes mellitus; TIRFM, total internal reflection fluorescence microscopy; VAMPs, Vesicle Associated Membrane Proteins; t-, target-; v-, vesicle-.
Figures



References
-
- Jahn R., Scheller R.H. SNAREs–engines for membrane fusion. Nature Reviews Molecular Cell Biology. 2006;7(9):631–643. - PubMed
-
- Tamori Y., Kawanishi M., Niki T., Shinoda H., Araki S., Okazawa H. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. The Journal of Biological Chemistry. 1998;273(31):19740–19746. - PubMed
-
- Tellam J.T., McIntosh S., James D.E. Molecular identification of two novel Munc-18 isoforms expressed in non-neuronal tissues. The Journal of Biological Chemistry. 1995;270(11):5857–5863. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

