Misfolding, Aggregation, and Disordered Segments in c-Abl and p53 in Human Cancer
- PMID: 25973395
- PMCID: PMC4413674
- DOI: 10.3389/fonc.2015.00097
Misfolding, Aggregation, and Disordered Segments in c-Abl and p53 in Human Cancer
Abstract
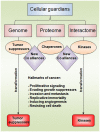
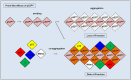
The current understanding of the molecular mechanisms that lead to cancer is not sufficient to explain the loss or gain of function in proteins related to tumorigenic processes. Among them, more than 100 oncogenes, 20-30 tumor-suppressor genes, and hundreds of genes participating in DNA repair and replication have been found to play a role in the origins of cancer over the last 25 years. The phosphorylation of serine, threonine, or tyrosine residues is a critical step in cellular growth and development and is achieved through the tight regulation of protein kinases. Phosphorylation plays a major role in eukaryotic signaling as kinase domains are found in 2% of our genes. The deregulation of kinase control mechanisms has disastrous consequences, often leading to gains of function, cell transformation, and cancer. The c-Abl kinase protein is one of the most studied targets in the fight against cancer and is a hotspot for drug development because it participates in several solid tumors and is the hallmark of chronic myelogenous leukemia. Tumor suppressors have the opposite effects. Their fundamental role in the maintenance of genomic integrity has awarded them a role as the guardians of DNA. Among the tumor suppressors, p53 is the most studied. The p53 protein has been shown to be a transcription factor that recognizes and binds to specific DNA response elements and activates gene transcription. Stress triggered by ionizing radiation or other mutagenic events leads to p53 phosphorylation and cell-cycle arrest, senescence, or programed cell death. The p53 gene is the most frequently mutated gene in cancer. Mutations in the DNA-binding domain are classified as class I or class II depending on whether substitutions occur in the DNA contact sites or in the protein core, respectively. Tumor-associated p53 mutations often lead to the loss of protein function, but recent investigations have also indicated gain-of-function mutations. The prion-like aggregation of mutant p53 is associated with loss-of-function, dominant-negative, and gain-of-function effects. In the current review, we focused on the most recent insights into the protein structure and function of the c-Abl and p53 proteins that will provide us guidance to understand the loss and gain of function of these misfolded tumor-associated proteins.
Keywords: cancer; kinases; misfolding; signaling; tumor suppressor.
Figures


Similar articles
-
Determination of cell fate by c-Abl activation in the response to DNA damage.Oncogene. 1998 Dec 24;17(25):3309-18. doi: 10.1038/sj.onc.1202571. Oncogene. 1998. PMID: 9916993 Review.
-
Gene methylation in gastric cancer.Clin Chim Acta. 2013 Sep 23;424:53-65. doi: 10.1016/j.cca.2013.05.002. Epub 2013 May 10. Clin Chim Acta. 2013. PMID: 23669186 Review.
-
Role for c-Abl tyrosine kinase in growth arrest response to DNA damage.Nature. 1996 Jul 18;382(6588):272-4. doi: 10.1038/382272a0. Nature. 1996. PMID: 8717045
-
Phosphorylation of the Mdm2 oncoprotein by the c-Abl tyrosine kinase regulates p53 tumor suppression and the radiosensitivity of mice.Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):15024-15029. doi: 10.1073/pnas.1611798114. Epub 2016 Dec 12. Proc Natl Acad Sci U S A. 2016. PMID: 27956626 Free PMC article.
-
c-ABL tyrosine kinase modulates p53-dependent p21 induction and ensuing cell fate decision in response to DNA damage.Cell Signal. 2014 Feb;26(2):444-52. doi: 10.1016/j.cellsig.2013.10.005. Epub 2013 Oct 28. Cell Signal. 2014. PMID: 24177958
Cited by
-
Confocal Spectroscopy to Study Dimerization, Oligomerization and Aggregation of Proteins: A Practical Guide.Int J Mol Sci. 2016 Apr 30;17(5):655. doi: 10.3390/ijms17050655. Int J Mol Sci. 2016. PMID: 27144560 Free PMC article. Review.
-
Amyloid aggregates induced by the p53-R280T mutation lead to loss of p53 function in nasopharyngeal carcinoma.Cell Death Dis. 2024 Jan 11;15(1):35. doi: 10.1038/s41419-024-06429-8. Cell Death Dis. 2024. PMID: 38212344 Free PMC article.
-
The absence of specific yeast heat-shock proteins leads to abnormal aggregation and compromised autophagic clearance of mutant Huntingtin proteins.PLoS One. 2018 Jan 18;13(1):e0191490. doi: 10.1371/journal.pone.0191490. eCollection 2018. PLoS One. 2018. PMID: 29346421 Free PMC article.
-
Aggregation and Prion-Like Properties of Misfolded Tumor Suppressors: Is Cancer a Prion Disease?Cold Spring Harb Perspect Biol. 2016 Oct 3;8(10):a023614. doi: 10.1101/cshperspect.a023614. Cold Spring Harb Perspect Biol. 2016. PMID: 27549118 Free PMC article. Review.
-
Neurodegeneration and Cancer: Where the Disorder Prevails.Sci Rep. 2015 Oct 23;5:15390. doi: 10.1038/srep15390. Sci Rep. 2015. PMID: 26493371 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

