Hsp90, the concertmaster: tuning transcription
- PMID: 25973397
- PMCID: PMC4412016
- DOI: 10.3389/fonc.2015.00100
Hsp90, the concertmaster: tuning transcription
Abstract
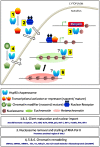
In the last decade, Hsp90 has emerged as a major regulator of cancer cell growth and proliferation. In cancer cells, it assists in giving maturation to oncogenic proteins including several kinases and transcription factors (TF). Recent studies have shown that apart from its chaperone activity, it also imparts regulation of transcription machinery and thereby alters the cellular physiology. Hsp90 and its co-chaperones modulate transcription at least at three different levels. In the first place, they alter the steady-state levels of certain TFs in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression. In this review, we discuss the role of Hsp90 in all the three aforementioned mechanisms of transcriptional control, taking examples from various model organisms with a special emphasis on cancer progression.
Keywords: Hsp90; cancer; chromatin modifiers; transcription; transcription factors.
Figures
Similar articles
-
Hsp90 as a "Chaperone" of the Epigenome: Insights and Opportunities for Cancer Therapy.Adv Cancer Res. 2016;129:107-40. doi: 10.1016/bs.acr.2015.09.003. Epub 2015 Nov 24. Adv Cancer Res. 2016. PMID: 26916003 Review.
-
Mechanisms of Hsp90 regulation.Biochem J. 2016 Aug 15;473(16):2439-52. doi: 10.1042/BCJ20160005. Biochem J. 2016. PMID: 27515256 Free PMC article. Review.
-
Hsp90: a chaperone for protein folding and gene regulation.Biochem Cell Biol. 2005 Dec;83(6):703-10. doi: 10.1139/o05-158. Biochem Cell Biol. 2005. PMID: 16333321 Review.
-
Targeting the Hsp90-Cdc37-client protein interaction to disrupt Hsp90 chaperone machinery.J Hematol Oncol. 2018 Apr 27;11(1):59. doi: 10.1186/s13045-018-0602-8. J Hematol Oncol. 2018. PMID: 29699578 Free PMC article. Review.
-
Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery.Exp Biol Med (Maywood). 2003 Feb;228(2):111-33. doi: 10.1177/153537020322800201. Exp Biol Med (Maywood). 2003. PMID: 12563018 Review.
Cited by
-
GALNT5 uaRNA promotes gastric cancer progression through its interaction with HSP90.Oncogene. 2018 Aug;37(33):4505-4517. doi: 10.1038/s41388-018-0266-4. Epub 2018 May 10. Oncogene. 2018. PMID: 29743591
-
Molecular docking performance evaluated on the D3R Grand Challenge 2015 drug-like ligand datasets.J Comput Aided Mol Des. 2016 Sep;30(9):829-839. doi: 10.1007/s10822-016-9983-3. Epub 2016 Oct 3. J Comput Aided Mol Des. 2016. PMID: 27699554
-
Proteomic comparison of selective breeding and growth hormone transgenesis in fish: Unique pathways to enhanced growth.J Proteomics. 2019 Feb 10;192:114-124. doi: 10.1016/j.jprot.2018.08.013. Epub 2018 Aug 25. J Proteomics. 2019. PMID: 30153513 Free PMC article.
-
LSD1 engages a corepressor complex for the activation of the estrogen receptor α by estrogen and cAMP.Nucleic Acids Res. 2016 Oct 14;44(18):8655-8670. doi: 10.1093/nar/gkw522. Epub 2016 Jun 20. Nucleic Acids Res. 2016. PMID: 27325688 Free PMC article.
-
A Rare Case of Human Diphallia Associated with Hypospadias.Case Rep Urol. 2018 Jun 13;2018:8293036. doi: 10.1155/2018/8293036. eCollection 2018. Case Rep Urol. 2018. PMID: 30009078 Free PMC article.
References
-
- Gress TM, Muller-Pillasch F, Weber C, Lerch MM, Friess H, Buchler M, et al. Differential expression of heat shock proteins in pancreatic carcinoma. Cancer Res (1994) 54:547–51. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

