Ketogenic Diet Impairs FGF21 Signaling and Promotes Differential Inflammatory Responses in the Liver and White Adipose Tissue
- PMID: 25973847
- PMCID: PMC4431718
- DOI: 10.1371/journal.pone.0126364
Ketogenic Diet Impairs FGF21 Signaling and Promotes Differential Inflammatory Responses in the Liver and White Adipose Tissue
Abstract
Background/hypothesis: Beside its beneficial effects on weight loss, ketogenic diet (KD) causes dyslipidemia, a pro-inflammatory state involved in the development of hepatic steatosis, glucose intolerance and insulin resistance, although the latter is still being debated. Additionally, KD is known to increase fibroblast growth factor 21 (FGF21) plasma levels. However, FGF21 cannot initiate its beneficial actions on metabolism in these conditions. We therefore hypothesized and tested in the present study that KD may impair FGF21 signaling.
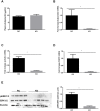
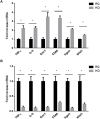
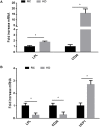
Methods/results: Using indirect calorimetry, we found that KD-fed mice exhibited higher energy expenditure than regular chow (RC)-fed mice associated with increased Ucp1 levels in white adipose tissue (WAT), along with increased plasma FGF21 levels. We then assessed the effect of KD on FGF21 signaling in both the liver and WAT. We found that Fgfr4 and Klb (β-klotho) were downregulated in the liver, while Fgfr1 was downregulated in WAT of KD-fed mice. Because inflammation could be one of the mechanisms linking KD to impaired FGF21 signaling, we measured the expression levels of inflammatory markers and macrophage accumulation in WAT and liver and found an increased inflammation and macrophage accumulation in the liver, but surprisingly, a reduction of inflammation in WAT.We also showed that KD enhances lipid accumulation in the liver, which may explain hepatic inflammation and impaired Fgfr4 and Klb expression. In contrast, import of lipids from the circulation was significantly reduced in WAT of KD-fed mice, as suggested by a downregulation of Lpl and Cd36. This was further associated with reduced inflammation in WAT.
Conclusion: Altogether, these results indicate that KD could be beneficial for a given tissue but deleterious for another.
Conflict of interest statement
Figures

References
-
- Jornayvaz FR, Samuel VT, Shulman GI. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annual review of nutrition. 2010;30:273–90. Epub 2010/07/22. 10.1146/annurev.nutr.012809.104726 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

