Efficient Radioisotope Energy Transfer by Gold Nanoclusters for Molecular Imaging
- PMID: 25973916
- PMCID: PMC12121654
- DOI: 10.1002/smll.201500907
Efficient Radioisotope Energy Transfer by Gold Nanoclusters for Molecular Imaging
Abstract
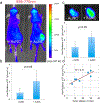
Beta-emitting isotopes Fluorine-18 and Yttrium-90 are tested for their potential to stimulate gold nanoclusters conjugated with blood serum proteins (AuNCs). AuNCs excited by either medical radioisotope are found to be highly effective ionizing radiation energy transfer mediators, suitable for in vivo optical imaging. AuNCs synthesized with protein templates convert beta-decaying radioisotope energy into tissue-penetrating optical signals between 620 and 800 nm. Optical signals are not detected from AuNCs incubated with Technetium-99m, a pure gamma emitter that is used as a control. Optical emission from AuNCs is not proportional to Cerenkov radiation, indicating that the energy transfer between the radionuclide and AuNC is only partially mediated by Cerenkov photons. A direct Coulombic interaction is proposed as a novel and significant mechanism of energy transfer between decaying radionuclides and AuNCs.
Keywords: cerenkov radiation; gold nanoclusters; imaging, radioisotope energy transfer; optical imaging; radioisotopes.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
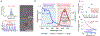


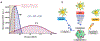
Similar articles
-
Steroid Probes Conjugated with Protein-Protected Gold Nanocluster: Specific and Rapid Fluorescence Imaging of Steroid Receptors in Target Cells.J Fluoresc. 2016 Jul;26(4):1239-48. doi: 10.1007/s10895-016-1811-6. Epub 2016 May 10. J Fluoresc. 2016. PMID: 27165037
-
Gold nanocluster-loaded hybrid albumin nanoparticles with fluorescence-based optical visualization and photothermal conversion for tumor detection/ablation.J Control Release. 2019 Jun 28;304:7-18. doi: 10.1016/j.jconrel.2019.04.036. Epub 2019 Apr 24. J Control Release. 2019. PMID: 31028785
-
Near infrared fluorescent trypsin stabilized gold nanoclusters as surface plasmon enhanced energy transfer biosensor and in vivo cancer imaging bioprobe.Anal Chem. 2013 Mar 19;85(6):3238-45. doi: 10.1021/ac303603f. Epub 2013 Mar 4. Anal Chem. 2013. PMID: 23413985
-
Water-Dispersible Gold Nanoclusters: Synthesis Strategies, Optical Properties, and Biological Applications.Chemistry. 2022 Feb 21;28(10):e202103736. doi: 10.1002/chem.202103736. Epub 2021 Dec 22. Chemistry. 2022. PMID: 34854510 Review.
-
Recent Advances in Biosensors Using Enzyme-Stabilized Gold Nanoclusters.Biosensors (Basel). 2024 Dec 24;15(1):2. doi: 10.3390/bios15010002. Biosensors (Basel). 2024. PMID: 39852053 Free PMC article. Review.
Cited by
-
Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy.ACS Nano. 2016 Apr 26;10(4):3918-35. doi: 10.1021/acsnano.6b01401. Epub 2016 Apr 8. ACS Nano. 2016. PMID: 27043181 Free PMC article. Review.
-
Second window near-infrared dosimeter (NIR2D) system for radiation dosimetry.Phys Med Biol. 2020 Aug 31;65(17):175013. doi: 10.1088/1361-6560/ab9b56. Phys Med Biol. 2020. PMID: 32869751 Free PMC article.
-
Radio-nanomaterials for biomedical applications: state of the art.Eur J Nanomed. 2016 Jul;8(3):151-170. doi: 10.1515/ejnm-2016-0011. Epub 2016 Feb 6. Eur J Nanomed. 2016. PMID: 27482194 Free PMC article.
-
Radionuclide-Activated Nanomaterials and Their Biomedical Applications.Angew Chem Int Ed Engl. 2019 Sep 16;58(38):13232-13252. doi: 10.1002/anie.201900594. Epub 2019 Jul 8. Angew Chem Int Ed Engl. 2019. PMID: 30779286 Free PMC article. Review.
-
Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy.Chem Soc Rev. 2016 Nov 21;45(23):6597-6626. doi: 10.1039/c6cs00271d. Chem Soc Rev. 2016. PMID: 27722328 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

