Slow breathing and hypoxic challenge: cardiorespiratory consequences and their central neural substrates
- PMID: 25973923
- PMCID: PMC4431729
- DOI: 10.1371/journal.pone.0127082
Slow breathing and hypoxic challenge: cardiorespiratory consequences and their central neural substrates
Abstract
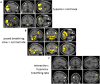
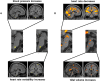
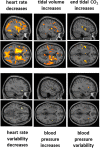
Controlled slow breathing (at 6/min, a rate frequently adopted during yoga practice) can benefit cardiovascular function, including responses to hypoxia. We tested the neural substrates of cardiorespiratory control in humans during volitional controlled breathing and hypoxic challenge using functional magnetic resonance imaging (fMRI). Twenty healthy volunteers were scanned during paced (slow and normal rate) breathing and during spontaneous breathing of normoxic and hypoxic (13% inspired O2) air. Cardiovascular and respiratory measures were acquired concurrently, including beat-to-beat blood pressure from a subset of participants (N = 7). Slow breathing was associated with increased tidal ventilatory volume. Induced hypoxia raised heart rate and suppressed heart rate variability. Within the brain, slow breathing activated dorsal pons, periaqueductal grey matter, cerebellum, hypothalamus, thalamus and lateral and anterior insular cortices. Blocks of hypoxia activated mid pons, bilateral amygdalae, anterior insular and occipitotemporal cortices. Interaction between slow breathing and hypoxia was expressed in ventral striatal and frontal polar activity. Across conditions, within brainstem, dorsal medullary and pontine activity correlated with tidal volume and inversely with heart rate. Activity in rostroventral medulla correlated with beat-to-beat blood pressure and heart rate variability. Widespread insula and striatal activity tracked decreases in heart rate, while subregions of insular cortex correlated with momentary increases in tidal volume. Our findings define slow breathing effects on central and cardiovascular responses to hypoxic challenge. They highlight the recruitment of discrete brainstem nuclei to cardiorespiratory control, and the engagement of corticostriatal circuitry in support of physiological responses that accompany breathing regulation during hypoxic challenge.
Conflict of interest statement
Figures

Similar articles
-
Hypoxic tachycardia is not a result of increased respiratory activity in healthy subjects.Exp Physiol. 2019 Apr;104(4):476-489. doi: 10.1113/EP087233. Epub 2019 Feb 12. Exp Physiol. 2019. PMID: 30672622
-
Modulation of spontaneous breathing via limbic/paralimbic-bulbar circuitry: an event-related fMRI study.Neuroimage. 2009 Sep;47(3):961-71. doi: 10.1016/j.neuroimage.2009.05.025. Epub 2009 May 18. Neuroimage. 2009. PMID: 19450692 Free PMC article.
-
Participation of the dorsal periaqueductal grey matter in the hypoxic ventilatory response in unanaesthetized rats.Acta Physiol (Oxf). 2014 Jul;211(3):528-37. doi: 10.1111/apha.12254. Epub 2014 Mar 10. Acta Physiol (Oxf). 2014. PMID: 24612700
-
Respiratory and cardiovascular responses to hypoxemia and the effects of anesthesia.Int Anesthesiol Clin. 1981 Fall;19(3):85-122. doi: 10.1097/00004311-198119030-00008. Int Anesthesiol Clin. 1981. PMID: 7026455 Review.
-
AMPK breathing and oxygen supply.Respir Physiol Neurobiol. 2019 Jul;265:112-120. doi: 10.1016/j.resp.2018.08.011. Epub 2018 Sep 19. Respir Physiol Neurobiol. 2019. PMID: 30243821 Review.
Cited by
-
Longitudinal single-subject neuroimaging study reveals effects of daily environmental, physiological, and lifestyle factors on functional brain connectivity.PLoS Biol. 2024 Oct 8;22(10):e3002797. doi: 10.1371/journal.pbio.3002797. eCollection 2024 Oct. PLoS Biol. 2024. PMID: 39378200 Free PMC article.
-
Online Questionnaire with Fibromyalgia Patients Reveals Correlations among Type of Pain, Psychological Alterations, and Effectiveness of Non-Pharmacological Therapies.Healthcare (Basel). 2022 Oct 9;10(10):1975. doi: 10.3390/healthcare10101975. Healthcare (Basel). 2022. PMID: 36292422 Free PMC article.
-
Towards real-world generalizability of a circuit for action-stopping.Nat Rev Neurosci. 2021 Sep;22(9):538-552. doi: 10.1038/s41583-021-00485-1. Epub 2021 Jul 29. Nat Rev Neurosci. 2021. PMID: 34326532 Free PMC article. Review.
-
A virtual reality-based mind-body approach to downregulate psychophysiological arousal in adolescent insomnia.Digit Health. 2022 Jun 15;8:20552076221107887. doi: 10.1177/20552076221107887. eCollection 2022 Jan-Dec. Digit Health. 2022. PMID: 35733879 Free PMC article.
-
What Has Neuroimaging Taught Us on the Neurobiology of Yoga? A Review.Front Integr Neurosci. 2020 Jul 8;14:34. doi: 10.3389/fnint.2020.00034. eCollection 2020. Front Integr Neurosci. 2020. PMID: 32733213 Free PMC article. Review.
References
-
- Bernardi L, Gabutti A, Porta C, Spicuzza L. Slowbreathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J Hypertens. 2001;19: 2221–2229. - PubMed
-
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, et al. Slowbreathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation. 2002;2: 143–145. - PubMed
-
- Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, et al. Slowbreathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46: 714–718. - PubMed
-
- Spicuzza L, Gabutti A, Porta C, Montano N, Bernardi L. Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet. 2000;356: 1495–1496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

