Optogenetic stimulation of mPFC pyramidal neurons as a conditioned stimulus supports associative learning in rats
- PMID: 25973929
- PMCID: PMC4431347
- DOI: 10.1038/srep10065
Optogenetic stimulation of mPFC pyramidal neurons as a conditioned stimulus supports associative learning in rats
Abstract
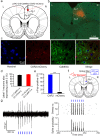
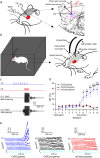
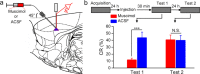
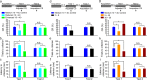
It is generally accepted that the associative learning occurs when a behaviorally neutral conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US) in close temporal proximity. Eyeblink conditioning (EBC) is a simple form of associative learning for motor responses. Specific activation of a population of cells may be an effective and sufficient CS for establishing EBC. However, there has been no direct evidence to support this hypothesis. Here, we show in rats that optogenetic activation of the right caudal mPFC pyramidal neurons as a CS is sufficient to support the acquisition of delay eyeblink conditioning (DEC). Interestingly, the associative memory was not stably expressed during the initial period of daily conditioning session even after the CR acquisition reached the asymptotic level. Finally, the intensity and consistency of the CS were found to be crucial factors in regulating the retrieval of the associative memory. These results may be of importance in understanding the neural cellular mechanisms underlying associative learning and the mechanisms underlying retrieval process of memory.
Figures

Similar articles
-
Medial Prefrontal Cortex-Pontine Nuclei Projections Modulate Suboptimal Cue-Induced Associative Motor Learning.Cereb Cortex. 2018 Mar 1;28(3):880-893. doi: 10.1093/cercor/bhw410. Cereb Cortex. 2018. PMID: 28077515 Free PMC article.
-
Transfer of classical eyeblink conditioning with electrical stimulation of mPFC or tone as conditioned stimulus in guinea pigs.Behav Brain Res. 2014 Nov 1;274:19-29. doi: 10.1016/j.bbr.2014.07.051. Epub 2014 Aug 10. Behav Brain Res. 2014. PMID: 25106738
-
Reevaluating the ability of cerebellum in associative motor learning.Sci Rep. 2019 Apr 15;9(1):6029. doi: 10.1038/s41598-019-42413-5. Sci Rep. 2019. PMID: 30988338 Free PMC article.
-
Neural substrates of eyeblink conditioning: acquisition and retention.Learn Mem. 2003 Nov-Dec;10(6):427-55. doi: 10.1101/lm.59603. Learn Mem. 2003. PMID: 14657256 Review.
-
The neural circuitry and molecular mechanisms underlying delay and trace eyeblink conditioning in mice.Behav Brain Res. 2015 Feb 1;278:307-14. doi: 10.1016/j.bbr.2014.10.006. Epub 2014 Oct 17. Behav Brain Res. 2015. PMID: 25448430 Review.
Cited by
-
Monocarboxylate Transporter 1 in the Medial Prefrontal Cortex Developmentally Expresses in Oligodendrocytes and Associates with Neuronal Amounts.Mol Neurobiol. 2017 Apr;54(3):2315-2326. doi: 10.1007/s12035-016-9820-7. Epub 2016 Mar 9. Mol Neurobiol. 2017. PMID: 26957300
-
Time-limited involvement of caudal anterior cingulate cortex in trace eyeblink conditioning retrieval is dependent on conditioned stimulus intensity.PLoS One. 2018 Jan 25;13(1):e0191320. doi: 10.1371/journal.pone.0191320. eCollection 2018. PLoS One. 2018. PMID: 29370235 Free PMC article.
-
Establishment and transfer of classical eyeblink conditioning using electrical microstimulation of the hippocampus as the conditioned stimulus.PLoS One. 2017 Jun 2;12(6):e0178502. doi: 10.1371/journal.pone.0178502. eCollection 2017. PLoS One. 2017. PMID: 28575003 Free PMC article.
-
Optogenetic Stimulation of the Anterior Cingulate Cortex Ameliorates Autistic-Like Behaviors in Rats Induced by Neonatal Isolation, Caudate Putamen as a Site for Alteration.Neuromolecular Med. 2019 Jun;21(2):132-142. doi: 10.1007/s12017-019-08526-w. Epub 2019 Feb 19. Neuromolecular Med. 2019. PMID: 30784006
-
Medial Prefrontal Cortex-Pontine Nuclei Projections Modulate Suboptimal Cue-Induced Associative Motor Learning.Cereb Cortex. 2018 Mar 1;28(3):880-893. doi: 10.1093/cercor/bhw410. Cereb Cortex. 2018. PMID: 28077515 Free PMC article.
References
-
- Christian K. M. & Thompson R. F. Neural substrates of eyeblink conditioning: acquisition and retention. Learn. Mem. 10, 427–455 (2003). - PubMed
-
- Christian K. M. & Thompson R. F. Long-term storage of an associative memory trace in the cerebellum. Behav. Neurosci. 119, 526–537 (2005). - PubMed
-
- Thompson R. F. In search of memory traces. Annu. Rev. Psychol. 56, 1–23 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

