The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- PMID: 25973932
- PMCID: PMC4431810
- DOI: 10.1371/journal.pgen.1005233
The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
Abstract
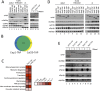
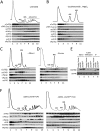
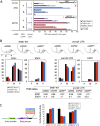
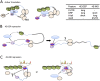
Translation initiation factor eIF4E mediates mRNA selection for protein synthesis via the mRNA 5'cap. A family of binding proteins, termed the 4E-BPs, interact with eIF4E to hinder ribosome recruitment. Mechanisms underlying mRNA specificity for 4E-BP control remain poorly understood. Saccharomyces cerevisiae 4E-BPs, Caf20p and Eap1p, each regulate an overlapping set of mRNAs. We undertook global approaches to identify protein and RNA partners of both 4E-BPs by immunoprecipitation of tagged proteins combined with mass spectrometry or next-generation sequencing. Unexpectedly, mass spectrometry indicated that the 4E-BPs associate with many ribosomal proteins. 80S ribosome and polysome association was independently confirmed and was not dependent upon interaction with eIF4E, as mutated forms of both Caf20p and Eap1p with disrupted eIF4E-binding motifs retain ribosome interaction. Whole-cell proteomics revealed Caf20p mutations cause both up and down-regulation of proteins and that many changes were independent of the 4E-binding motif. Investigations into Caf20p mRNA targets by immunoprecipitation followed by RNA sequencing revealed a strong association between Caf20p and mRNAs involved in transcription and cell cycle processes, consistent with observed cell cycle phenotypes of mutant strains. A core set of over 500 Caf20p-interacting mRNAs comprised of both eIF4E-dependent (75%) and eIF4E-independent targets (25%), which differ in sequence attributes. eIF4E-independent mRNAs share a 3' UTR motif. Caf20p binds all tested motif-containing 3' UTRs. Caf20p and the 3'UTR combine to influence ERS1 mRNA polysome association consistent with Caf20p contributing to translational control. Finally ERS1 3'UTR confers Caf20-dependent repression of expression to a heterologous reporter gene. Taken together, these data reveal conserved features of eIF4E-dependent Caf20p mRNA targets and uncover a novel eIF4E-independent mode of Caf20p binding to mRNAs that extends the regulatory role of Caf20p in the mRNA-specific repression of protein synthesis beyond its interaction with eIF4E.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

