The Downregulation of MiR-182 Is Associated with the Growth and Invasion of Osteosarcoma Cells through the Regulation of TIAM1 Expression
- PMID: 25973950
- PMCID: PMC4431740
- DOI: 10.1371/journal.pone.0121175
The Downregulation of MiR-182 Is Associated with the Growth and Invasion of Osteosarcoma Cells through the Regulation of TIAM1 Expression
Retraction in
-
Retraction: The Downregulation of MiR-182 Is Associated with the Growth and Invasion of Osteosarcoma Cells through the Regulation of TIAM1 Expression.PLoS One. 2023 Apr 21;18(4):e0283115. doi: 10.1371/journal.pone.0283115. eCollection 2023. PLoS One. 2023. PMID: 37083557 Free PMC article. No abstract available.
Abstract
Background: Osteosarcoma is the most common primary bone malignancy in children and young adults. Increasing results suggest that discovery of microRNAs (miRNAs) might provide a novel therapeutical target for osteosarcoma.
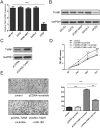
Methods: MiR-182 expression level in osteosarcoma cell lines and tissues were assayed by qRT-PCR. MiRNA mimics or inhibitor were transfected for up-regulation or down-regulation of miR-182 expression. Cell function was assayed by CCK8, migration assay and invasion assay. The target genes of miR-182 were predicated by bioinformatics algorithm (TargetScan Human).
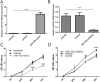
Results: MiR-182 was down-regulated in osteosarcoma tissues and cell lines. Overexpression of miR-182 inhibited tumor growth, migration and invasion. Subsequent investigation revealed that TIAM1 was a direct and functional target of miR-182 in osteosarcoma cells. Overexpression of miR-182 impaired TIAM1-induced inhibition of proliferation and invasion in osteosarcoma cells.
Conclusions: Down-expression of miR-182 in osteosarcoma promoted tumor growth, migration and invasion by targeting TIAM1. MiR-182 might act as a tumor suppressor gene whose down-regulation contributes to the progression and metastasis of osteosarcoma, providing a potential therapy target for osteosarcoma patients.
Conflict of interest statement
Figures



Similar articles
-
miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9.Asian Pac J Cancer Prev. 2013;14(6):3681-4. doi: 10.7314/apjcp.2013.14.6.3681. Asian Pac J Cancer Prev. 2013. PMID: 23886165
-
Knockdown of HCG18 Inhibits Cell Viability, Migration and Invasion in Pediatric Osteosarcoma by Targeting miR-188-5p/FOXC1 Axis.Mol Biotechnol. 2021 Sep;63(9):807-817. doi: 10.1007/s12033-021-00343-6. Epub 2021 May 26. Mol Biotechnol. 2021. PMID: 34041718
-
miR-454 is down-regulated in osteosarcomas and suppresses cell proliferation and invasion by directly targeting c-Met.Cell Prolif. 2015 Jun;48(3):348-55. doi: 10.1111/cpr.12187. Epub 2015 Apr 16. Cell Prolif. 2015. PMID: 25880599 Free PMC article.
-
miR-34 inhibits growth and promotes apoptosis of osteosarcoma in nude mice through targetly regulating TGIF2 expression.Biosci Rep. 2018 Jun 12;38(3):BSR20180078. doi: 10.1042/BSR20180078. Print 2018 Jun 29. Biosci Rep. 2018. Retraction in: Biosci Rep. 2024 Feb 28;44(2):BSR-2018-0078_RET. doi: 10.1042/BSR-2018-0078_RET. PMID: 29895719 Free PMC article. Retracted.
-
miRNA signatures in childhood sarcomas and their clinical implications.Clin Transl Oncol. 2019 Dec;21(12):1583-1623. doi: 10.1007/s12094-019-02104-z. Epub 2019 Apr 4. Clin Transl Oncol. 2019. PMID: 30949930 Review.
Cited by
-
EBV and 1q Gains Affect Gene and miRNA Expression in Burkitt Lymphoma.Viruses. 2023 Aug 25;15(9):1808. doi: 10.3390/v15091808. Viruses. 2023. PMID: 37766215 Free PMC article.
-
Down regulation of miR-30a-5p and miR-182-5p in gastric cancer: Clinical impact and survival analysis.Biochem Biophys Rep. 2021 Jul 20;27:101079. doi: 10.1016/j.bbrep.2021.101079. eCollection 2021 Sep. Biochem Biophys Rep. 2021. PMID: 34355069 Free PMC article.
-
Analysis of non‑alcoholic fatty liver disease microRNA expression spectra in rat liver tissues.Mol Med Rep. 2018 Sep;18(3):2669-2680. doi: 10.3892/mmr.2018.9268. Epub 2018 Jul 9. Mol Med Rep. 2018. PMID: 30015905 Free PMC article.
-
MicroRNA Regulation of the Small Rho GTPase Regulators-Complexities and Opportunities in Targeting Cancer Metastasis.Cancers (Basel). 2020 Apr 28;12(5):1092. doi: 10.3390/cancers12051092. Cancers (Basel). 2020. PMID: 32353968 Free PMC article. Review.
-
miR‑328‑3p enhances the radiosensitivity of osteosarcoma and regulates apoptosis and cell viability via H2AX.Oncol Rep. 2018 Feb;39(2):545-553. doi: 10.3892/or.2017.6112. Epub 2017 Nov 27. Oncol Rep. 2018. PMID: 29207178 Free PMC article.
References
-
- Li G, Cai M, Fu D, Chen K, Sun M, Cai Z, et al.. Heat shock protein 90B1 plays an oncogenic role and is a target of microRNA-223 in human osteosarcoma. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;30(6):1481–90. - PubMed
-
- Hasei J, Sasaki T, Tazawa H, Osaki S, Yamakawa Y, Kunisada T, et al.. Dual programmed cell death pathways induced by p53 transactivation overcome resistance to oncolytic adenovirus in human osteosarcoma cells. Molecular cancer therapeutics. 2013;12(3):314–25. doi: 10.1158/1535-7163.MCT-12-0869 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

