Fob1 and Fob2 Proteins Are Virulence Determinants of Rhizopus oryzae via Facilitating Iron Uptake from Ferrioxamine
- PMID: 25974051
- PMCID: PMC4431732
- DOI: 10.1371/journal.ppat.1004842
Fob1 and Fob2 Proteins Are Virulence Determinants of Rhizopus oryzae via Facilitating Iron Uptake from Ferrioxamine
Abstract
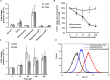
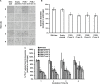
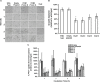
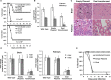
Dialysis patients with chronic renal failure receiving deferoxamine for treating iron overload are uniquely predisposed for mucormycosis, which is most often caused by Rhizopus oryzae. Although the deferoxamine siderophore is not secreted by Mucorales, previous studies established that Rhizopus species utilize iron from ferrioxamine (iron-rich form of deferoxamine). Here we determined that the CBS domain proteins of Fob1 and Fob2 act as receptors on the cell surface of R. oryzae during iron uptake from ferrioxamine. Fob1 and Fob2 cell surface expression was induced in the presence of ferrioxamine and bound radiolabeled ferrioxamine. A R. oryzae strain with targeted reduced Fob1/Fob2 expression was impaired for iron uptake, germinating, and growing on medium with ferrioxamine as the sole source of iron. This strain also exhibited reduced virulence in a deferoxamine-treated, but not the diabetic ketoacidotic (DKA), mouse model of mucormycosis. The mechanism by which R. oryzae obtains iron from ferrioxamine involves the reductase/permease uptake system since the growth on ferrioxamine supplemented medium is associated with elevated reductase activity and the use of the ferrous chelator bathophenanthroline disulfonate abrogates iron uptake and growth on medium supplemented with ferrioxamine as a sole source of iron. Finally, R. oryzae mutants with reduced copies of the high affinity iron permease (FTR1) or with decreased FTR1 expression had an impaired iron uptake from ferrioxamine in vitro and reduced virulence in the deferoxamine-treated mouse model of mucormycosis. These two receptors appear to be conserved in Mucorales, and can be the subject of future novel therapy to maintain the use of deferoxamine for treating iron-overload.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis.Mol Microbiol. 2010 Aug;77(3):587-604. doi: 10.1111/j.1365-2958.2010.07234.x. Epub 2010 Jun 1. Mol Microbiol. 2010. PMID: 20545847 Free PMC article.
-
Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily.Microbiology (Reading). 1998 Dec;144 ( Pt 12):3455-3462. doi: 10.1099/00221287-144-12-3455. Microbiology (Reading). 1998. PMID: 9884238
-
Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus.Biochem Pharmacol. 1994 May 18;47(10):1843-50. doi: 10.1016/0006-2952(94)90314-x. Biochem Pharmacol. 1994. PMID: 8204101
-
Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment.Curr Opin Infect Dis. 2008 Dec;21(6):620-5. doi: 10.1097/QCO.0b013e3283165fd1. Curr Opin Infect Dis. 2008. PMID: 18978530 Free PMC article. Review.
-
[Iron and invasive fungal infection].Rev Iberoam Micol. 2013 Oct-Dec;30(4):217-25. doi: 10.1016/j.riam.2013.04.002. Epub 2013 May 14. Rev Iberoam Micol. 2013. PMID: 23684655 Review. Spanish.
Cited by
-
Mucor circinelloides Thrives inside the Phagosome through an Atf-Mediated Germination Pathway.mBio. 2019 Feb 5;10(1):e02765-18. doi: 10.1128/mBio.02765-18. mBio. 2019. PMID: 30723131 Free PMC article.
-
Mucorales and Mucormycosis: Recent Insights and Future Prospects.J Fungi (Basel). 2023 Mar 9;9(3):335. doi: 10.3390/jof9030335. J Fungi (Basel). 2023. PMID: 36983503 Free PMC article. Review.
-
Animal Models to Study Mucormycosis.J Fungi (Basel). 2019 Mar 27;5(2):27. doi: 10.3390/jof5020027. J Fungi (Basel). 2019. PMID: 30934788 Free PMC article. Review.
-
Advancements of fish-derived peptides for mucormycosis: a novel strategy to treat diabetic compilation.Mol Biol Rep. 2023 Dec;50(12):10485-10507. doi: 10.1007/s11033-023-08882-8. Epub 2023 Nov 2. Mol Biol Rep. 2023. PMID: 37917415 Review.
-
Future Prospects and Challenges in Mucormycosis Research.J Fungi (Basel). 2025 Jul 22;11(8):545. doi: 10.3390/jof11080545. J Fungi (Basel). 2025. PMID: 40863497 Free PMC article.
References
-
- Ibrahim AS, Edwards JE Jr., Filler SG, Spellberg B (2011) Mucormycosis and Entomophtoramycosis (Zygomycosis) In: Kauffman CA PP, Sobel JD, Dismukes WE, 2nd Ed., editor. Essentials of Clinical Mycology. New York: Springer; pp. 265–280.
-
- Kontoyiannis DP, Lewis RE (2006) Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am 20: 581–607, vi. - PubMed
-
- Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E (2004) Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma 45: 1351–1360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

