The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole
- PMID: 25974303
- PMCID: PMC4435723
- DOI: 10.1016/j.chom.2015.04.003
The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole
Abstract
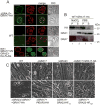
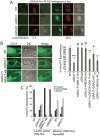
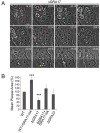
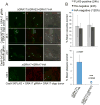
Toxoplasma gondii is a protozoan pathogen in the phylum Apicomplexa that resides within an intracellular parasitophorous vacuole (PV) that is selectively permeable to small molecules through unidentified mechanisms. We have identified GRA17 as a Toxoplasma-secreted protein that localizes to the parasitophorous vacuole membrane (PVM) and mediates passive transport of small molecules across the PVM. GRA17 is related to the putative Plasmodium translocon protein EXP2 and conserved across PV-residing Apicomplexa. The PVs of GRA17-deficient parasites have aberrant morphology, reduced permeability to small molecules, and structural instability. GRA17-deficient parasites proliferate slowly and are avirulent in mice. These GRA17-deficient phenotypes are rescued by complementation with Plasmodium EXP2. GRA17 functions synergistically with a related protein, GRA23. Exogenous expression of GRA17 or GRA23 alters the membrane conductance properties of Xenopus oocytes in a manner consistent with a large non-selective pore. Thus, GRA17 and GRA23 provide a molecular basis for PVM permeability and nutrient access.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, et al. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe. 2013;13:489–500. - PubMed
-
- Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, Hussain T, Kieffer-Jaquinod S, Coute Y, Pelloux H, Tardieux I, et al. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med. 2013;210:2071–2086. - PMC - PubMed
-
- Coppens I. Exploitation of auxotrophies and metabolic defects in Toxoplasma as therapeutic approaches. Int J Parasitol. 2014;44:109–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1TR000124/TR/NCATS NIH HHS/United States
- R01-AI064616/AI/NIAID NIH HHS/United States
- T32 GM007287/GM/NIGMS NIH HHS/United States
- 1R21AI114930/AI/NIAID NIH HHS/United States
- T32-AI07323/AI/NIAID NIH HHS/United States
- F31 AI104170/AI/NIAID NIH HHS/United States
- R21 AI114930/AI/NIAID NIH HHS/United States
- R01 AI080621/AI/NIAID NIH HHS/United States
- T32 AI007323/AI/NIAID NIH HHS/United States
- R01 AI064616/AI/NIAID NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- R03 AI101749/AI/NIAID NIH HHS/United States
- R01 HL062465/HL/NHLBI NIH HHS/United States
- DP5 OD017892/OD/NIH HHS/United States
- F31-AI104170/AI/NIAID NIH HHS/United States
- R01-HL062465/HL/NHLBI NIH HHS/United States
- 1-DP5-OD017892/OD/NIH HHS/United States
- 5R01-AI080621/AI/NIAID NIH HHS/United States
- R03-AI101749/AI/NIAID NIH HHS/United States
- 5-T32-GM007287-33/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

