Microenvironmental control of stem cell fate in intestinal homeostasis and disease
- PMID: 25974319
- PMCID: PMC4744721
- DOI: 10.1002/path.4563
Microenvironmental control of stem cell fate in intestinal homeostasis and disease
Abstract
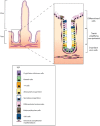
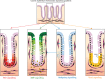
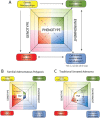
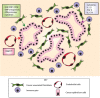
The conventional model of intestinal epithelial architecture describes a unidirectional tissue organizational hierarchy with stem cells situated at the crypt base and daughter cells proliferating and terminally differentiating as they progress along the vertical (crypt-luminal) axis. In this model, the fate of a cell that has left the niche is determined and its lifespan limited. Evidence is accumulating to suggest that stem cell control and daughter cell fate determination is not solely an intrinsic, cell autonomous property but is heavily influenced by the microenvironment including paracrine, mesenchymal, and endogenous epithelial morphogen gradients. Recent research suggests that in intestinal homeostasis, stem cells transit reversibly between states of variable competence in the niche. Furthermore, selective pressures that disrupt the homeostatic balance, such as intestinal inflammation or morphogen dysregulation, can cause committed progenitor cells and even some differentiated cells to regain stem cell properties. Importantly, it has been recently shown that this disruption of cell fate determination can lead to somatic mutation and neoplastic transformation of cells situated outside the crypt base stem cell niche. This paper reviews the exciting developments in the study of stem cell dynamics in homeostasis, intestinal regeneration, and carcinogenesis, and explores the implications for human disease and cancer therapies.
Keywords: chronic inflammation; colon; neoplasia.
© 2015 Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures

References
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 1974; 141 : 461–479. - PubMed
-
- Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 1977; 269: 518‐521. - PubMed
-
- Scoville DH, Sato T, He XC, et al. Current view: intestinal stem cells and signaling. Gastroenterology 2008; 134: 849‐864. - PubMed
-
- Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 2011; 474: 318‐326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

