Two Hydroxyproline Galactosyltransferases, GALT5 and GALT2, Function in Arabinogalactan-Protein Glycosylation, Growth and Development in Arabidopsis
- PMID: 25974423
- PMCID: PMC4431829
- DOI: 10.1371/journal.pone.0125624
Two Hydroxyproline Galactosyltransferases, GALT5 and GALT2, Function in Arabinogalactan-Protein Glycosylation, Growth and Development in Arabidopsis
Abstract
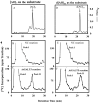
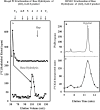
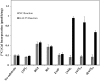
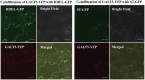
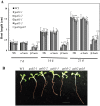
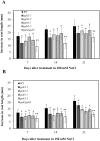
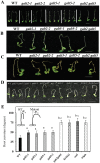
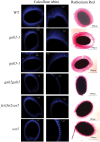
Hydroxyproline-O-galactosyltransferase (GALT) initiates O-glycosylation of arabinogalactan-proteins (AGPs). We previously characterized GALT2 (At4g21060), and now report on functional characterization of GALT5 (At1g74800). GALT5 was identified using heterologous expression in Pichia and an in vitro GALT assay. Product characterization showed GALT5 specifically adds galactose to hydroxyproline in AGP protein backbones. Functions of GALT2 and GALT5 were elucidated by phenotypic analysis of single and double mutant plants. Allelic galt5 and galt2 mutants, and particularly galt2 galt5 double mutants, demonstrated lower GALT activities and reductions in β-Yariv-precipitated AGPs compared to wild type. Mutant plants showed pleiotropic growth and development phenotypes (defects in root hair growth, root elongation, pollen tube growth, flowering time, leaf development, silique length, and inflorescence growth), which were most severe in the double mutants. Conditional mutant phenotypes were also observed, including salt-hypersensitive root growth and root tip swelling as well as reduced inhibition of pollen tube growth and root growth in response to β-Yariv reagent. These mutants also phenocopy mutants for an AGP, SOS5, and two cell wall receptor-like kinases, FEI1 and FEI2, which exist in a genetic signaling pathway. In summary, GALT5 and GALT2 function as redundant GALTs that control AGP O-glycosylation, which is essential for normal growth and development.
Conflict of interest statement
Figures

References
-
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3: 1–30. - PubMed
-
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A (2011) Plant Cell Walls. (New York: Garland Science, Taylor & Francis Group; ).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

