HaloTag technology: a versatile platform for biomedical applications
- PMID: 25974629
- PMCID: PMC4482335
- DOI: 10.1021/acs.bioconjchem.5b00191
HaloTag technology: a versatile platform for biomedical applications
Abstract
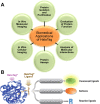
Exploration of protein function and interaction is critical for discovering links among genomics, proteomics, and disease state; yet, the immense complexity of proteomics found in biological systems currently limits our investigational capacity. Although affinity and autofluorescent tags are widely employed for protein analysis, these methods have been met with limited success because they lack specificity and require multiple fusion tags and genetic constructs. As an alternative approach, the innovative HaloTag protein fusion platform allows protein function and interaction to be comprehensively analyzed using a single genetic construct with multiple capabilities. This is accomplished using a simplified process, in which a variable HaloTag ligand binds rapidly to the HaloTag protein (usually linked to the protein of interest) with high affinity and specificity. In this review, we examine all current applications of the HaloTag technology platform for biomedical applications, such as the study of protein isolation and purification, protein function, protein-protein and protein-DNA interactions, biological assays, in vitro cellular imaging, and in vivo molecular imaging. In addition, novel uses of the HaloTag platform are briefly discussed along with potential future applications.
Figures



References
-
- Robinson C. V.; Sali A.; Baumeister W. (2007) The molecular sociology of the cell. Nature 450, 973–982. - PubMed
-
- Hanash S. (2003) Disease proteomics. Nature 422, 226–232. - PubMed
-
- Hanash S. M.; Pitteri S. J.; Faca V. M. (2008) Mining the plasma proteome for cancer biomarkers. Nature 452, 571–579. - PubMed
-
- Kolch W.; Mischak H.; Pitt A. R. (2005) The molecular make-up of a tumour: proteomics in cancer research. Clin. Sci. 108, 369–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

