Pathological and Clinical Spectrum of Progressive Supranuclear Palsy: With Special Reference to Astrocytic Tau Pathology
- PMID: 25974705
- PMCID: PMC8029026
- DOI: 10.1111/bpa.12265
Pathological and Clinical Spectrum of Progressive Supranuclear Palsy: With Special Reference to Astrocytic Tau Pathology
Abstract
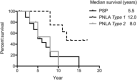
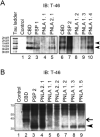
Progressive supranuclear palsy (PSP) is a four-repeat tauopathy with tau-positive, argyrophilic tuft-shaped astrocytes (TAs). We performed a pathological and clinical investigation in 40 consecutive autopsied Japanese patients with pathological diagnoses of PSP or PSP-like disease. Unequivocal TAs were present in 22 cases, all of which were confirmed to be PSP. Such TAs were hardly detected in the other 18 cases, which instead exhibited tau-positive, argyrophilic astrocytes, appearing as comparatively small clusters with central nuclei of irregularly shaped, coarse structures (equivocal TAs). Cluster analysis of the distribution pattern of tau-related pathology for these 18 cases identified two subgroups, pallido-nigro-luysian atrophy (PNLA) Type 1 (n = 9) and Type 2 (n = 9), the former being distinguished from the latter by the presence of tau-related lesions in the motor cortex, pontine nucleus and cerebellar dentate nucleus in addition to the severely affected PNL system. The duration from symptom onset until becoming wheelchair-bound was significantly longer in PNLA Type 1. Immunoblotting of samples from the three disease conditions revealed band patterns of low-molecular-mass tau fragments at ∼35 kDa. These findings shed further light on the wide pathological and clinical spectrum of four-repeat tauopathy, representing PSP in the broad sense rather than classical PSP.
Keywords: glial tau pathology; pallido-nigro-luysian atrophy; progressive supranuclear palsy; tauopathy; tuft-shaped astrocyte.
© 2015 International Society of Neuropathology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






References
-
- Ahmed Z, Josephs KA, Gonzalez J, DelleDonne A, Dickson DW (2008) Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido‐nigro‐luysial degeneration and axonal dystrophy. Brain 131:460–472. - PubMed
-
- Ahmed Z, Tabrizi SJ, Li A, Houlden H, Sailer A, Lees AJ et al (2010) A Huntington's disease phenocopy characterized by pallido‐nigro‐luysian degeneration with brain iron accumulation and p62‐positive glial inclusions. Neuropathol Appl Neurobiol 36:551–557. - PubMed
-
- Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K et al (2004) Identification of amino‐terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79. - PubMed
-
- Arima K (2006) Ultrastructural characteristics of tau filaments in tauopathies: immuno‐electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 26:475–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

