Experimental approaches to identify small RNAs and their diverse roles in bacteria--what we have learnt in one decade of MicA research
- PMID: 25974745
- PMCID: PMC4618604
- DOI: 10.1002/mbo3.263
Experimental approaches to identify small RNAs and their diverse roles in bacteria--what we have learnt in one decade of MicA research
Abstract
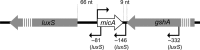
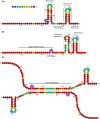
Nowadays the identification of small RNAs (sRNAs) and characterization of their role within regulatory networks takes a prominent place in deciphering complex bacterial phenotypes. Compared to the study of other components of bacterial cells, this is a relatively new but fast-growing research field. Although reports on new sRNAs appear regularly, some sRNAs are already subject of research for a longer time. One of such sRNAs is MicA, a sRNA best described for its role in outer membrane remodeling, but probably having a much broader function than anticipated. An overview of what we have learnt from MicA led to the conclusion that even for this well-described sRNA, we still do not have the overall picture. More general, the story of MicA might become an experimental lead for unraveling the many sRNAs with unknown functions. In this review, three important topics in the sRNA field are covered, exemplified from the perspective of MicA: (i) identification of new sRNAs, (ii) target identification and unraveling the biological function, (iii) structural analysis. The complex mechanisms of action of MicA deliver some original insights in the sRNA field which includes the existence of dimer formation or simultaneous cis and trans regulation, and might further inspire the understanding of the function of other sRNAs.
Keywords: Conservation; MicA; sRNA; structure; target identification.
© 2015 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Small RNA-mediated regulation in bacteria: A growing palette of diverse mechanisms.Gene. 2018 May 20;656:60-72. doi: 10.1016/j.gene.2018.02.068. Epub 2018 Mar 1. Gene. 2018. PMID: 29501814 Review.
-
sRNA Target Prediction Organizing Tool (SPOT) Integrates Computational and Experimental Data To Facilitate Functional Characterization of Bacterial Small RNAs.mSphere. 2019 Jan 30;4(1):e00561-18. doi: 10.1128/mSphere.00561-18. mSphere. 2019. PMID: 30700509 Free PMC article.
-
Bacterial sRNAs: regulation in stress.Int J Med Microbiol. 2013 Jul;303(5):217-29. doi: 10.1016/j.ijmm.2013.04.002. Epub 2013 Apr 11. Int J Med Microbiol. 2013. PMID: 23660175 Review.
-
Small RNAs in streptococci.RNA Biol. 2012 Apr;9(4):414-26. doi: 10.4161/rna.20104. Epub 2012 Apr 1. RNA Biol. 2012. PMID: 22546939 Review.
-
Integration of Bacterial Small RNAs in Regulatory Networks.Annu Rev Biophys. 2017 May 22;46:131-148. doi: 10.1146/annurev-biophys-070816-034058. Annu Rev Biophys. 2017. PMID: 28532217 Review.
Cited by
-
Direct interaction of small non-coding RNAs CjNC140 and CjNC110 optimizes expression of key pathogenic phenotypes of Campylobacter jejuni.mBio. 2023 Aug 31;14(4):e0083323. doi: 10.1128/mbio.00833-23. Epub 2023 Jul 6. mBio. 2023. PMID: 37409826 Free PMC article.
-
Small Regulatory RNAs of Rickettsia conorii.Sci Rep. 2016 Nov 11;6:36728. doi: 10.1038/srep36728. Sci Rep. 2016. PMID: 27834404 Free PMC article.
-
Evidence for Involvement of the Salmonella enterica Z-Ring Assembly Factors ZapA and ZapB in Resistance to Bile.Front Microbiol. 2021 Feb 25;12:647305. doi: 10.3389/fmicb.2021.647305. eCollection 2021. Front Microbiol. 2021. PMID: 33717045 Free PMC article.
-
An Intertwined Network of Regulation Controls Membrane Permeability Including Drug Influx and Efflux in Enterobacteriaceae.Microorganisms. 2020 Jun 1;8(6):833. doi: 10.3390/microorganisms8060833. Microorganisms. 2020. PMID: 32492979 Free PMC article. Review.
-
Role of Non-coding Regulatory RNA in the Virulence of Human Pathogenic Vibrios.Front Microbiol. 2017 Jan 11;7:2160. doi: 10.3389/fmicb.2016.02160. eCollection 2016. Front Microbiol. 2017. PMID: 28123382 Free PMC article. Review.
References
-
- Ades SE. Regulation by destruction: design of the sigmaE envelope stress response. Curr. Opin. Microbiol. 2008;11:535–540. - PubMed
-
- Altuvia S. Identification of bacterial small non-coding RNAs: experimental approaches. Curr. Opin. Microbiol. 2007;10:257–261. - PubMed
-
- Andrade JM, Pobre V, Silva IJ, Domingues S. Arraiano CM. The role of 3’-5’ exoribonucleases in RNA degradation. Prog. Mol. Biol. Trans. Sci. 2009;85:187–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

