Antifibrotic therapies in the liver
- PMID: 25974903
- PMCID: PMC5743222
- DOI: 10.1055/s-0035-1550055
Antifibrotic therapies in the liver
Abstract
Significant progress has been made in understanding the principles underlying the development of liver fibrosis. This includes appreciating its dynamic nature, the importance of active fibrolysis in fibrosis regression, and the plasticity of cell populations endowing them with fibrogenic or fibrolytic properties. This is complemented by an increasing array of therapeutic targets with known roles in the progression or regression of fibrosis. With a key role for fibrosis in determining clinical outcomes and encouraging data from recently Food and Drug Administration-approved antifibrotics for pulmonary fibrosis, the development and validation of antifibrotic therapies has taken center stage in translational hepatology. In addition to summarizing the recent progress in antifibrotic therapies, the authors discuss some of the challenges ahead, such as achieving a better understanding of the interindividual heterogeneity of the fibrotic response, how to match interventions with the ideal patient population, and the development of better noninvasive methods to assess the dynamics of fibrogenesis and fibrolysis. Together, these advances will permit a better targeting and dose titration of individualized therapies. Finally, the authors discuss combination therapy with different antifibrotics as possibly the most potent approach for treating fibrosis in the liver.
Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Figures

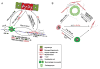
References
-
- Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5(167):167s. r1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

