Heavy Metal Ion Regulation of Gene Expression: MECHANISMS BY WHICH LEAD INHIBITS OSTEOBLASTIC BONE-FORMING ACTIVITY THROUGH MODULATION OF THE Wnt/β-CATENIN SIGNALING PATHWAY
- PMID: 25975268
- PMCID: PMC4505064
- DOI: 10.1074/jbc.M114.629204
Heavy Metal Ion Regulation of Gene Expression: MECHANISMS BY WHICH LEAD INHIBITS OSTEOBLASTIC BONE-FORMING ACTIVITY THROUGH MODULATION OF THE Wnt/β-CATENIN SIGNALING PATHWAY
Abstract
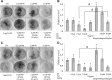
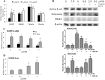
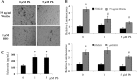
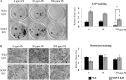
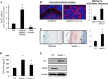
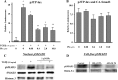
Exposure to lead (Pb) from environmental sources remains an overlooked and serious public health risk. Starting in childhood, Pb in the skeleton can disrupt epiphyseal plate function, constrain the growth of long bones, and prevent attainment of a high peak bone mass, all of which will increase susceptibility to osteoporosis later in life. We hypothesize that the effects of Pb on bone mass, in part, come from depression of Wnt/β-catenin signaling, a critical anabolic pathway for osteoblastic bone formation. In this study, we show that depression of Wnt signaling by Pb is due to increased sclerostin levels in vitro and in vivo. Downstream activation of the β-catenin pathway using a pharmacological inhibitor of GSK-3β ameliorates the Pb inhibition of Wnt signaling activity in the TOPGAL reporter mouse. The effect of Pb was determined to be dependent on sclerostin expression through use of the SOST gene knock-out mice, which are resistant to Pb-induced trabecular bone loss and maintain their mechanical bone strength. Moreover, isolated bone marrow cells from the sclerostin null mice show improved bone formation potential even after exposure to Pb. Also, our data suggest that the TGFβ canonical signaling pathway is the mechanism by which Pb controls sclerostin production. Taken together these results support our hypothesis that the osteoporotic-like phenotype observed after Pb exposure is, in part, regulated through modulation of the Wnt/β-catenin pathway.
Keywords: TGF-β signaling; Wnt signaling; bone; gene expression; lead toxicity; osteoblast; osteoporosis; sclerostin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Fluoride promotes osteoblastic differentiation through canonical Wnt/β-catenin signaling pathway.Toxicol Lett. 2014 Feb 10;225(1):34-42. doi: 10.1016/j.toxlet.2013.11.029. Epub 2013 Dec 1. Toxicol Lett. 2014. PMID: 24300170
-
GLP-1 promotes osteogenic differentiation of human ADSCs via the Wnt/GSK-3β/β-catenin pathway.Mol Cell Endocrinol. 2020 Sep 15;515:110921. doi: 10.1016/j.mce.2020.110921. Epub 2020 Jun 29. Mol Cell Endocrinol. 2020. PMID: 32615283
-
Daphnetin ameliorates glucocorticoid-induced osteoporosis via activation of Wnt/GSK-3β/β-catenin signaling.Toxicol Appl Pharmacol. 2020 Dec 15;409:115333. doi: 10.1016/j.taap.2020.115333. Epub 2020 Nov 7. Toxicol Appl Pharmacol. 2020. PMID: 33171191
-
Wnt signaling as a therapeutic target for bone diseases.Expert Opin Ther Targets. 2009 Apr;13(4):485-96. doi: 10.1517/14728220902841961. Expert Opin Ther Targets. 2009. PMID: 19335070 Free PMC article. Review.
-
Involvement of WNT/β-catenin signaling in the treatment of osteoporosis.Calcif Tissue Int. 2013 Aug;93(2):121-32. doi: 10.1007/s00223-013-9749-z. Epub 2013 Jun 11. Calcif Tissue Int. 2013. PMID: 23748710 Review.
Cited by
-
A One-Two Punch to Bone: Assessing the Combined Impact of Lead and a High-Fat Diet.Environ Health Perspect. 2015 Oct;123(10):A264. doi: 10.1289/ehp.123-A264. Environ Health Perspect. 2015. PMID: 26421523 Free PMC article. No abstract available.
-
Environmental Factors Impacting Bone-Relevant Chemokines.Front Endocrinol (Lausanne). 2017 Feb 14;8:22. doi: 10.3389/fendo.2017.00022. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28261155 Free PMC article.
-
Prenatal lead (Pb) exposure is associated with differential placental DNA methylation and hydroxymethylation in a human population.Epigenetics. 2022 Dec;17(13):2404-2420. doi: 10.1080/15592294.2022.2126087. Epub 2022 Sep 23. Epigenetics. 2022. PMID: 36148884 Free PMC article.
-
Nucleic Acid Aptamers Protect Against Lead (Pb(II)) Toxicity.bioRxiv [Preprint]. 2024 Mar 31:2024.03.28.587288. doi: 10.1101/2024.03.28.587288. bioRxiv. 2024. Update in: N Biotechnol. 2024 Nov 25;83:36-45. doi: 10.1016/j.nbt.2024.06.004. PMID: 38585880 Free PMC article. Updated. Preprint.
-
Sclerostin activity plays a key role in the negative effect of glucocorticoid signaling on osteoblast function in mice.Bone Res. 2017 May 9;5:17013. doi: 10.1038/boneres.2017.13. eCollection 2017. Bone Res. 2017. PMID: 28529816 Free PMC article.
References
-
- Gruber H. E., Gonick H. C., Khalil-Manesh F., Sanchez T. V., Motsinger S., Meyer M., Sharp C. F. (1997) Osteopenia induced by long-term, low- and high-level exposure of the adult rat to lead. Miner. Electrolyte Metab. 23, 65–73 - PubMed
-
- Klein R. F., Wiren K. M. (1993) Regulation of osteoblastic gene expression by lead. Endocrinology 132, 2531–2537 - PubMed
-
- Sauk J. J., Smith T., Silbergeld E. K., Fowler B. A., Somerman M. J. (1992) Lead inhibits secretion of osteonectin/SPARC without significantly altering collagen or Hsp47 production in osteoblast-like ROS 17/2.8 cells. Toxicol. Appl. Pharmacol. 116, 240–247 - PubMed
-
- Angle C. R., Thomas D. J., Swanson S. A. (1990) Lead inhibits the basal and stimulated responses of a rat osteoblast-like cell line ROS 17/2.8 to 1 α,25-dihydroxyvitamin D3 and IGF-I. Toxicol. Appl. Pharmacol. 103, 281–287 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

