Mechanistic Aspects of Folded Protein Transport by the Twin Arginine Translocase (Tat)
- PMID: 25975269
- PMCID: PMC4505407
- DOI: 10.1074/jbc.R114.626820
Mechanistic Aspects of Folded Protein Transport by the Twin Arginine Translocase (Tat)
Abstract
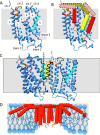
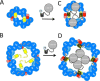
The twin arginine translocase (Tat) transports folded proteins of widely varying size across ionically tight membranes with only 2-3 components of machinery and the proton motive force. Tat operates by a cycle in which the receptor complex combines with the pore-forming component to assemble a new translocase for each substrate. Recent data on component and substrate organization in the receptor complex and on the structure of the pore complex inform models for translocase assembly and translocation. A translocation mechanism involving local transient bilayer rupture is discussed.
Keywords: Signal peptide; TatA; TatC; Thylakoid membrane; Twin Arginine Translocation; chloroplast; intracellular trafficking; membrane; organelle; protein translocation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Cline K., Theg S. (2007) The Sec and Tat protein translocation pathways in chloroplasts. in The Enzymes: Molecular Machines Involved in Protein Transport across Cellular Membranes (Dalbey R. E., Koehler C. M., Tamanoi F., eds), pp. 463–492, Academic Press, New York
-
- Palmer T., Berks B. C. (2012) The twin-arginine translocation (Tat) protein export pathway. Nat. Rev. Microbiol. 10, 483–496 - PubMed
-
- Berks B. C. (2015) The twin-arginine protein translocation pathway. Annu. Rev. Biochem., 84, 843–864 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

