Loss of the Mono-ADP-ribosyltransferase, Tiparp, Increases Sensitivity to Dioxin-induced Steatohepatitis and Lethality
- PMID: 25975270
- PMCID: PMC4505429
- DOI: 10.1074/jbc.M115.660100
Loss of the Mono-ADP-ribosyltransferase, Tiparp, Increases Sensitivity to Dioxin-induced Steatohepatitis and Lethality
Abstract
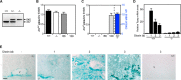
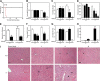
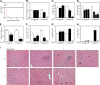
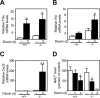
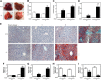
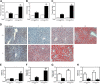
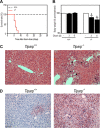
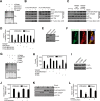
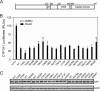
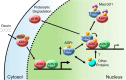
The aryl hydrocarbon receptor (AHR) mediates the toxic effects of the environmental contaminant dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin; TCDD). Dioxin causes a range of toxic responses, including hepatic damage, steatohepatitis, and a lethal wasting syndrome; however, the mechanisms are still unknown. Here, we show that the loss of TCDD-inducible poly(ADP-ribose) polymerase (Tiparp), an ADP-ribosyltransferase and AHR repressor, increases sensitivity to dioxin-induced toxicity, steatohepatitis, and lethality. Tiparp(-/-) mice given a single injection of 100 μg/kg dioxin did not survive beyond day 5; all Tiparp(+/+) mice survived the 30-day treatment. Dioxin-treated Tiparp(-/-) mice exhibited increased liver steatosis and hepatotoxicity. Tiparp ADP-ribosylated AHR but not its dimerization partner, the AHR nuclear translocator, and the repressive effects of TIPARP on AHR were reversed by the macrodomain containing mono-ADP-ribosylase MACROD1 but not MACROD2. These results reveal previously unidentified roles for Tiparp, MacroD1, and ADP-ribosylation in AHR-mediated steatohepatitis and lethality in response to dioxin.
Keywords: ADP-ribosylation; aryl hydrocarbon receptor (AhR) (AHR); dioxin; gene expression; toxicity.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Nebert D. W., Puga A., Vasiliou V. (1993) Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann. N.Y. Acad. Sci. 685, 624–640 - PubMed
-
- Denis M., Cuthill S., Wikström A. C., Poellinger L., Gustafsson J. A. (1988) Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem. Biophys. Res. Commun. 155, 801–807 - PubMed
-
- Perdew G. H. (1988) Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. 263, 13802–13805 - PubMed
-
- Carver L. A., Bradfield C. A. (1997) Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J. Biol. Chem. 272, 11452–11456 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

