Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker
- PMID: 25975510
- PMCID: PMC4559283
- DOI: 10.1016/j.tins.2015.04.003
Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker
Abstract
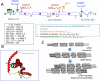
Glial fibrillary acidic protein (GFAP) is an intermediate filament (IF) III protein uniquely found in astrocytes in the central nervous system (CNS), non-myelinating Schwann cells in the peripheral nervous system (PNS), and enteric glial cells. GFAP mRNA expression is regulated by several nuclear-receptor hormones, growth factors, and lipopolysaccharides (LPSs). GFAP is also subject to numerous post-translational modifications (PTMs), while GFAP mutations result in protein deposits known as Rosenthal fibers in Alexander disease. GFAP gene activation and protein induction appear to play a critical role in astroglial cell activation (astrogliosis) following CNS injuries and neurodegeneration. Emerging evidence also suggests that, following traumatic brain and spinal cord injuries and stroke, GFAP and its breakdown products are rapidly released into biofluids, making them strong candidate biomarkers for such neurological disorders.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Eng LF, et al. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem. Res. 2000;25:1439–1451. - PubMed
-
- Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nature Reviews Gastroenterology & Hepatology. 2012;9:625–632. - PubMed
-
- Su A, editor. Dataset: GeneAtlas U133A, gcrma. [Online] 2012 Available: http://biogps.org/dataset/1/. [Accessed: 28-Mar-2015]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

