Intestinal Antigen-Presenting Cells: Key Regulators of Immune Homeostasis and Inflammation
- PMID: 25976247
- PMCID: PMC4483458
- DOI: 10.1016/j.ajpath.2015.02.024
Intestinal Antigen-Presenting Cells: Key Regulators of Immune Homeostasis and Inflammation
Abstract
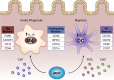
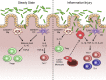
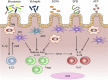
The microbiota that populate the mammalian intestine are critical for proper host physiology, yet simultaneously pose a potential danger. Intestinal antigen-presenting cells, namely macrophages and dendritic cells (DCs), are integral components of the mucosal innate immune system that maintain co-existence with the microbiota in face of this constant threat. Intestinal macrophages and DCs integrate signals from the microenvironment to orchestrate innate and adaptive immune responses that ultimately lead to durable tolerance of the microbiota. Tolerance is not a default response, however, because macrophages and DCs remain poised to vigorously respond to pathogens that breach the epithelial barrier. In this review, we summarize the salient features of macrophages and DCs in the healthy and inflamed intestine and discuss how signals from the microbiota can influence their function.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages.Mucosal Immunol. 2008 Nov;1(6):460-9. doi: 10.1038/mi.2008.61. Epub 2008 Sep 17. Mucosal Immunol. 2008. PMID: 19079213 Free PMC article. Review.
-
Essential immunologic orchestrators of intestinal homeostasis.Sci Immunol. 2018 Feb 9;3(20):eaao1605. doi: 10.1126/sciimmunol.aao1605. Sci Immunol. 2018. PMID: 29440266 Free PMC article. Review.
-
Dendritic cell-epithelial cell crosstalk in the gut.Immunol Rev. 2014 Jul;260(1):118-28. doi: 10.1111/imr.12181. Immunol Rev. 2014. PMID: 24942686 Review.
-
Phenotypic and functional profiling of mouse intestinal antigen presenting cells.J Immunol Methods. 2015 Jun;421:20-26. doi: 10.1016/j.jim.2015.03.023. Epub 2015 Apr 17. J Immunol Methods. 2015. PMID: 25891794 Free PMC article. Review.
-
Regulatory T cells and immune tolerance in the intestine.Cold Spring Harb Perspect Biol. 2013 Jul 1;5(7):a018341. doi: 10.1101/cshperspect.a018341. Cold Spring Harb Perspect Biol. 2013. PMID: 23818502 Free PMC article. Review.
Cited by
-
Homeostatic PPARα Signaling Limits Inflammatory Responses to Commensal Microbiota in the Intestine.J Immunol. 2016 Jun 1;196(11):4739-49. doi: 10.4049/jimmunol.1501489. Epub 2016 Apr 25. J Immunol. 2016. PMID: 27183583 Free PMC article.
-
EpCAM Is Essential to Maintaining the Immune Homeostasis of Intestines via Keeping the Expression of pIgR in the Intestinal Epithelium of Mice.Front Immunol. 2022 Apr 13;13:843378. doi: 10.3389/fimmu.2022.843378. eCollection 2022. Front Immunol. 2022. PMID: 35493520 Free PMC article.
-
Kynurenines as a Novel Target for the Treatment of Inflammatory Disorders.Cells. 2024 Jul 26;13(15):1259. doi: 10.3390/cells13151259. Cells. 2024. PMID: 39120289 Free PMC article. Review.
-
Transcriptomics of chicken cecal tonsils and intestine after infection with low pathogenic avian influenza virus H9N2.Sci Rep. 2021 Oct 14;11(1):20462. doi: 10.1038/s41598-021-99182-3. Sci Rep. 2021. PMID: 34650121 Free PMC article.
-
Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis.Front Immunol. 2019 Mar 6;10:360. doi: 10.3389/fimmu.2019.00360. eCollection 2019. Front Immunol. 2019. PMID: 30894857 Free PMC article. Review.
References
-
- Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
-
- Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. - PubMed
-
- Mowat A.M., Agace W.W. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. - PubMed
-
- Bain C.C., Mowat A.M. Intestinal macrophages: specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494–2498. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

