Physical activity for smoking cessation in pregnancy: randomised controlled trial
- PMID: 25976288
- PMCID: PMC4431606
- DOI: 10.1136/bmj.h2145
Physical activity for smoking cessation in pregnancy: randomised controlled trial
Abstract
Objective: To determine the effectiveness of a physical activity intervention for smoking cessation during pregnancy.
Design: Parallel group, randomised controlled, multicentre trial.
Setting: 13 hospitals in England, April 2009 to January 2014.
Participants: 789 pregnant smokers, aged 16-50 years and at 10-24 weeks' gestation, who smoked at least one cigarette daily and were prepared to quit smoking one week after enrollment were randomised (1:1); 785 were included in the intention to treat analyses, with 392 assigned to the physical activity group.
Interventions: Interventions began one week before a target quit date. Participants were randomised to six weekly sessions of behavioural support for smoking cessation (control) or to this support plus 14 sessions combining supervised treadmill exercise and physical activity consultations.
Main outcome measures: The primary outcome was continuous smoking abstinence from the target quit date until end of pregnancy, validated by exhaled carbon monoxide or salivary cotinine levels. To assess adherence, levels of moderate-vigorous intensity physical activity were self reported and in a 11.5% (n=90) random subsample of participants, physical activity was objectively measured by an accelerometer.
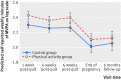
Results: No significant difference was found in rates of smoking abstinence at end of pregnancy between the physical activity and control groups (8% v 6%; odds ratio 1.21, 95% confidence interval 0.70 to 2.10). For the physical activity group compared with the control group, there was a 40% (95% confidence interval 13% to 73%), 34% (6% to 69%), and 46% (12% to 91%) greater increase in self reported minutes carrying out physical activity per week from baseline to one week, four weeks, and six weeks post-quit day, respectively. According to the accelerometer data there was no significant difference in physical activity levels between the groups. Participants attended a median of four treatment sessions in the intervention group and three in the control group. Adverse events and birth outcomes were similar between the two groups, except for significantly more caesarean births in the control group than in the physical activity group (29% v 21%, P=0.023).
Conclusion: Adding a physical activity intervention to behavioural smoking cessation support for pregnant women did not increase cessation rates at end of pregnancy. During pregnancy, physical activity is not recommended for smoking cessation but remains indicated for general health benefits. Trial registration Current Controlled Trials ISRCTN48600346.
© Ussher et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Trials on physical activity for smoking cessation in pregnancy missed a trick or two.BMJ. 2015 Jun 30;350:h3554. doi: 10.1136/bmj.h3554. BMJ. 2015. PMID: 26126757 No abstract available.
-
Authors' reply to Braillon and Bewley.BMJ. 2015 Jun 30;350:h3555. doi: 10.1136/bmj.h3555. BMJ. 2015. PMID: 26126981 No abstract available.
References
-
- Kallen K. The impact of maternal smoking during pregnancy on delivery outcome. Eur J Public Health 2001;11:329-33. - PubMed
-
- Rogers JM. Tobacco and pregnancy. Reprod Toxicol 2009;28:152-60. - PubMed
-
- Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev 2007;83:713-20. - PubMed
-
- NHS Information Centre. Infant feeding survey 2010: early results. NHS Information Centre for Health and Social Care, 2011.
-
- Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy—pregnancy risk assessment monitoring system, United States, 40 sites, 2000-2010. MMWR Surveill Summ 2013;62:1-19. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
