Lack of aprataxin impairs mitochondrial functions via downregulation of the APE1/NRF1/NRF2 pathway
- PMID: 25976310
- PMCID: PMC4512623
- DOI: 10.1093/hmg/ddv183
Lack of aprataxin impairs mitochondrial functions via downregulation of the APE1/NRF1/NRF2 pathway
Abstract
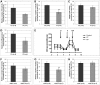
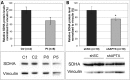
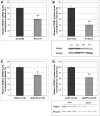
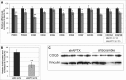
Ataxia oculomotor apraxia type 1 (AOA1) is an autosomal recessive disease caused by mutations in APTX, which encodes the DNA strand-break repair protein aprataxin (APTX). CoQ10 deficiency has been identified in fibroblasts and muscle of AOA1 patients carrying the common W279X mutation, and aprataxin has been localized to mitochondria in neuroblastoma cells, where it enhances preservation of mitochondrial function. In this study, we show that aprataxin deficiency impairs mitochondrial function, independent of its role in mitochondrial DNA repair. The bioenergetics defect in AOA1-mutant fibroblasts and APTX-depleted Hela cells is caused by decreased expression of SDHA and genes encoding CoQ biosynthetic enzymes, in association with reductions of APE1, NRF1 and NRF2. The biochemical and molecular abnormalities in APTX-depleted cells are recapitulated by knockdown of APE1 in Hela cells and are rescued by overexpression of NRF1/2. Importantly, pharmacological upregulation of NRF1 alone by 5-aminoimidazone-4-carboxamide ribonucleotide does not rescue the phenotype, which, in contrast, is reversed by the upregulation of NRF2 by rosiglitazone. Accordingly, we propose that the lack of aprataxin causes reduction of the pathway APE1/NRF1/NRF2 and their target genes. Our findings demonstrate a critical role of APTX in transcription regulation of mitochondrial function and the pathogenesis of AOA1 via a novel pathomechanistic pathway, which may be relevant to other neurodegenerative diseases.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

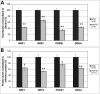
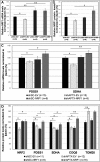
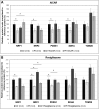
Similar articles
-
Human AP endonuclease/redox factor APE1/ref-1 modulates mitochondrial function after oxidative stress by regulating the transcriptional activity of NRF1.Free Radic Biol Med. 2012 Jul 15;53(2):237-48. doi: 10.1016/j.freeradbiomed.2012.04.002. Epub 2012 May 11. Free Radic Biol Med. 2012. PMID: 22580151
-
Aprataxin, poly-ADP ribose polymerase 1 (PARP-1) and apurinic endonuclease 1 (APE1) function together to protect the genome against oxidative damage.Hum Mol Genet. 2009 Nov 1;18(21):4102-17. doi: 10.1093/hmg/ddp359. Epub 2009 Jul 30. Hum Mol Genet. 2009. PMID: 19643912
-
Ataxia with oculomotor apraxia type1 (AOA1): novel and recurrent aprataxin mutations, coenzyme Q10 analyses, and clinical findings in Italian patients.Neurogenetics. 2011 Aug;12(3):193-201. doi: 10.1007/s10048-011-0281-x. Epub 2011 Apr 5. Neurogenetics. 2011. PMID: 21465257
-
Short-patch single-strand break repair in ataxia oculomotor apraxia-1.Biochem Soc Trans. 2009 Jun;37(Pt 3):577-81. doi: 10.1042/BST0370577. Biochem Soc Trans. 2009. PMID: 19442253 Review.
-
Early-onset ataxia with ocular motor apraxia and hypoalbuminemia/ataxia with oculomotor apraxia 1.Adv Exp Med Biol. 2010;685:21-33. doi: 10.1007/978-1-4419-6448-9_3. Adv Exp Med Biol. 2010. PMID: 20687492 Review.
Cited by
-
Mechanisms and Therapeutic Effects of Benzoquinone Ring Analogs in Primary CoQ Deficiencies.Antioxidants (Basel). 2022 Mar 30;11(4):665. doi: 10.3390/antiox11040665. Antioxidants (Basel). 2022. PMID: 35453349 Free PMC article. Review.
-
Neurological disorders associated with DNA strand-break processing enzymes.Mech Ageing Dev. 2017 Jan;161(Pt A):130-140. doi: 10.1016/j.mad.2016.07.009. Epub 2016 Jul 25. Mech Ageing Dev. 2017. PMID: 27470939 Free PMC article. Review.
-
Chemical Inhibition of Apurinic-Apyrimidinic Endonuclease 1 Redox and DNA Repair Functions Affects the Inflammatory Response via Different but Overlapping Mechanisms.Front Cell Dev Biol. 2021 Sep 20;9:731588. doi: 10.3389/fcell.2021.731588. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34616737 Free PMC article.
-
Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid.Sci Rep. 2015 Dec 2;5:17536. doi: 10.1038/srep17536. Sci Rep. 2015. PMID: 26625948 Free PMC article.
-
Targeting NRF2, Regulator of Antioxidant System, to Sensitize Glioblastoma Neurosphere Cells to Radiation-Induced Oxidative Stress.Oxid Med Cell Longev. 2020 Jun 15;2020:2534643. doi: 10.1155/2020/2534643. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32617133 Free PMC article.
References
-
- Inoue N., Izumi K., Mawatari S., Shida K., Kuroiwa Y. (1971) Congenital ocular motor apraxia and cerebellar degeneration: report of two cases. Rinsho Shinkeigaku, 11, 855–861.
-
- Uekawa K., Yuasa T., Kawasaki S., Makibuchi T., Ideta T. (1992) A hereditary ataxia associated with hypoalbuminemia and hyperlipidemia—a variant form of Friedreich's disease or a new clinical entity? Rinsho Shinkeigaku, 32, 1067–1074. - PubMed
-
- Le Ber I., Moreira M.C., Rivaud-Pechoux S., Chamayou C., Ochsner F., Kuntzer T., Tardieu M., Said G., Habert M.O., Demarquay G., et al. (2003) Cerebellar ataxia with oculomotor apraxia type 1: clinical and genetic studies. Brain, 126, 2761–2772. - PubMed
-
- Tranchant C., Fleury M., Moreira M.C., Koenig M., Warter J.M. (2003) Phenotypic variability of aprataxin gene mutations. Neurology, 60, 868–870. - PubMed
-
- Castellotti B., Mariotti C., Rimoldi M., Fancellu R., Plumari M., Caimi S., Uziel G., Nardocci N., Moroni I., Zorzi G., et al. (2011) Ataxia with oculomotor apraxia type1 (AOA1): novel and recurrent aprataxin mutations, coenzyme Q10 analyses, and clinical findings in Italian patients. Neurogenetics, 12, 193–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

