Effect of chemical modifications on allergenic potency of peanut proteins
- PMID: 25976435
- PMCID: PMC4405596
- DOI: 10.2500/aap.2015.36.3840
Effect of chemical modifications on allergenic potency of peanut proteins
Abstract
Background: Modification of native peanut extracts could reduce adverse effects of peanut immunotherapy.
Objective: We sought to compare native and chemically modified crude peanut extract (CPE) and major peanut allergens Ara h 2 and Ara h 6 in a mediator-release assay based on the rat basophilic leukemia (RBL) cell line transfected with human Fcε receptor.
Methods: Native Ara h 2/6 was reduced and alkylated (RA), with or without additional glutaraldehyde treatment (RAGA). CPE was reduced and alkylated. Sera of subjects with peanut allergy (16 males; median age 7 years) were used for overnight RBL-passive sensitization. Cells were stimulated with 0.1 pg/mL to 10 μg/mL of peanut. β-N-acetylhexosaminidase release (NHR) was used as a marker of RBL degranulation, expressed as a percentage of total degranulation caused by Triton X.
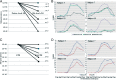
Results: Median peanut-specific immunoglobulin E was 233 kUA/L. Nineteen subjects were responders, NHR ≥ 10% in the mediator release assay. Responders had reduced NHR by RA and RAGA compared with the native Ara h 2/6. Modification resulted in a later onset of activation by 10- to 100-fold in concentration and a lowering of the maximum release. Modified RA-Ara h 2/6 and RAGA-Ara h 2/6 caused significantly lower maximum mediator release than native Ara h 2/6, at protein concentrations 0.1, 1, and 10 ng/mL (p < 0.001, < 0.001, and < 0.001, respectively, for RA; and < 0.001, 0.026, and 0.041, respectively, for RAGA). RA-CPE caused significantly lower maximum NHR than native CPE, at protein concentration 1 ng/mL (p < 0.001) and 10 ng/mL (p < 0.002). Responders had high rAra h 2 immunoglobulin E (mean, 61.1 kUA/L; p < 0.001) and higher NHR in mediator release assay to native Ara h 2/6 than CPE, which indicates that Ara h 2/6 were the most relevant peanut allergens in these responders.
Conclusions: Chemical modification of purified native Ara h 2 and Ara h 6 reduced mediator release in an in vitro assay ∼100-fold, which indicates decreased allergenicity for further development of the alternative candidate for safe peanut immunotherapy.
Conflict of interest statement
The authors have no conflicts of interest to declare that pertain to this article
Figures
Comment in
-
For the patient.Allergy Asthma Proc. 2015 May-Jun;36(3):236. doi: 10.2500/aap.2015.36.3856. Allergy Asthma Proc. 2015. PMID: 25976443 Free PMC article. No abstract available.
References
-
- Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 133:291–307, 2014. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol 107:191–193, 2001. - PubMed
-
- Dyer AA, Rivkina V, Perumal D, et al. Epidemiology of childhood peanut allergy. Allergy Asthma Proc 36:58–64, 2015. - PubMed
-
- Sampson HA, Aceves S, Bock SA, et al. Food allergy: A practice parameter update-2014. J Allergy Clin Immunol 134:1016–1025, 2014. - PubMed
-
- Nelson HS, Lahr J, Rule R, et al. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol 99:744–751, 1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

