Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus
- PMID: 25976618
- PMCID: PMC4430984
- DOI: 10.1186/s13041-015-0120-3
Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus
Abstract
Background: Chronic treatment with selective serotonin (5-HT) reuptake inhibitors (SSRIs) facilitates adult neurogenesis and reverses the state of maturation in mature granule cells (GCs) in the dentate gyrus (DG) of the hippocampus. Recent studies have suggested that the 5-HT4 receptor is involved in both effects. However, it is largely unknown how the 5-HT4 receptor mediates neurogenic effects in the DG and, how the neurogenic and dematuration effects of SSRIs interact with each other.
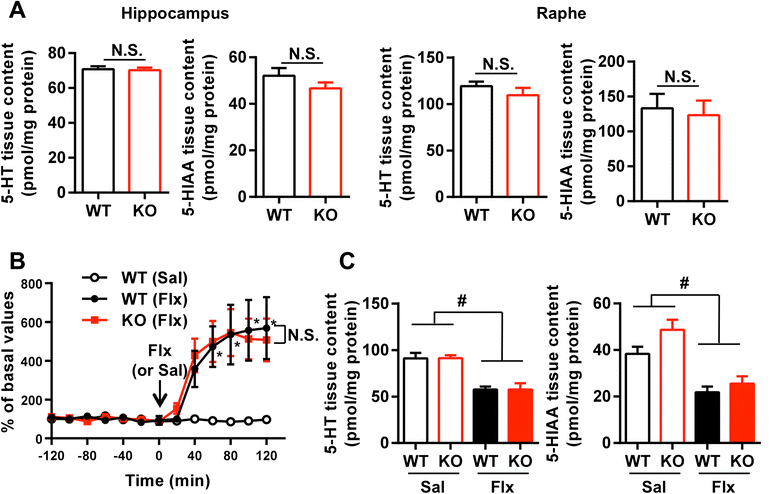
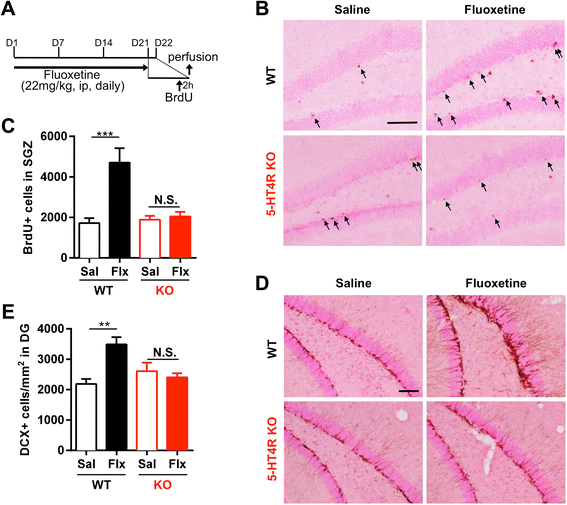
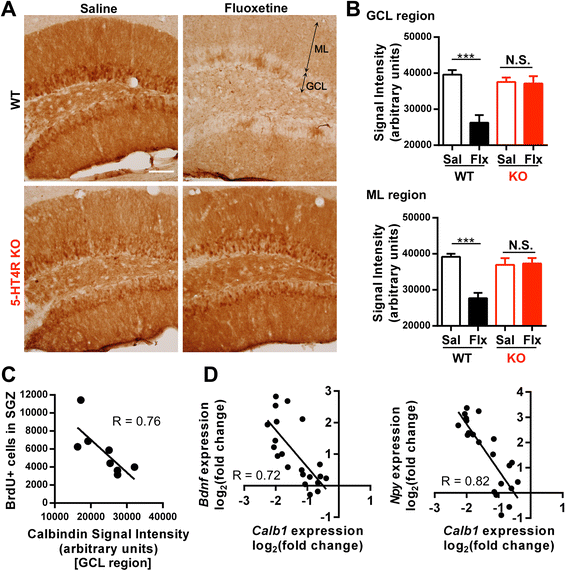
Results: We addressed these issues using 5-HT4 receptor knockout (5-HT4R KO) mice. Expression of the 5-HT4 receptor was detected in mature GCs but not in neuronal progenitors of the DG. We found that chronic treatment with the SSRI fluoxetine significantly increased cell proliferation and the number of doublecortin-positive cells in the DG of wild-type mice, but not in 5-HT4R KO mice. We then examined the correlation between the increased neurogenesis and the dematuration of GCs. As reported previously, reduced expression of calbindin in the DG, as an index of dematuration, by chronic fluoxetine treatment was observed in wild-type mice but not in 5-HT4R KO mice. The proliferative effect of fluoxetine was inversely correlated with the expression level of calbindin in the DG. The expression of neurogenic factors in the DG, such as brain derived neurotrophic factor (Bdnf), was also associated with the progression of dematuration. These results indicate that the neurogenic effects of fluoxetine in the DG are closely associated with the progression of dematuration of GCs. In contrast, the DG in which neurogenesis was impaired by irradiation still showed significant reduction of calbindin expression by chronic fluoxetine treatment, suggesting that dematuration of GCs by fluoxetine does not require adult neurogenesis in the DG.
Conclusions: We demonstrated that the 5-HT4 receptor plays an important role in fluoxetine-induced adult neurogenesis in the DG in addition to GC dematuration, and that these phenomena are closely associated. Our results suggest that 5-HT4 receptor-mediated phenotypic changes, including dematuration in mature GCs, underlie the neurogenic effect of SSRIs in the DG, providing new insight into the cellular mechanisms of the neurogenic actions of SSRIs in the hippocampus.
Figures



Similar articles
-
Fluoxetine induces input-specific hippocampal dendritic spine remodeling along the septotemporal axis in adulthood and middle age.Hippocampus. 2015 Nov;25(11):1429-46. doi: 10.1002/hipo.22464. Epub 2015 May 2. Hippocampus. 2015. PMID: 25850664 Free PMC article.
-
5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response.Nat Neurosci. 2015 Nov;18(11):1606-16. doi: 10.1038/nn.4116. Epub 2015 Sep 21. Nat Neurosci. 2015. PMID: 26389840 Free PMC article.
-
Behavioral destabilization induced by the selective serotonin reuptake inhibitor fluoxetine.Mol Brain. 2011 Mar 16;4:12. doi: 10.1186/1756-6606-4-12. Mol Brain. 2011. PMID: 21410937 Free PMC article.
-
The Effect of Serotonin-Targeting Antidepressants on Neurogenesis and Neuronal Maturation of the Hippocampus Mediated via 5-HT1A and 5-HT4 Receptors.Front Cell Neurosci. 2017 May 16;11:142. doi: 10.3389/fncel.2017.00142. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28559799 Free PMC article. Review.
-
Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise.Brain Res Bull. 2018 Oct;143:181-193. doi: 10.1016/j.brainresbull.2018.09.002. Epub 2018 Sep 17. Brain Res Bull. 2018. PMID: 30236533 Review.
Cited by
-
5-HT4 Receptors Are Not Involved in the Effects of Fluoxetine in the Corticosterone Model of Depression.ACS Chem Neurosci. 2021 Jun 2;12(11):2036-2044. doi: 10.1021/acschemneuro.1c00158. Epub 2021 May 11. ACS Chem Neurosci. 2021. PMID: 33974408 Free PMC article.
-
Antidepressant-like effects of standardized gypenosides: involvement of brain-derived neurotrophic factor signaling in hippocampus.Psychopharmacology (Berl). 2016 Sep;233(17):3211-21. doi: 10.1007/s00213-016-4357-z. Epub 2016 Jul 6. Psychopharmacology (Berl). 2016. PMID: 27385417
-
The multifaceted effects of fluoxetine treatment on cognitive functions.Front Pharmacol. 2024 Jul 16;15:1412420. doi: 10.3389/fphar.2024.1412420. eCollection 2024. Front Pharmacol. 2024. PMID: 39081952 Free PMC article. Review.
-
History and progress of hypotheses and clinical trials for Alzheimer's disease.Signal Transduct Target Ther. 2019 Aug 23;4:29. doi: 10.1038/s41392-019-0063-8. eCollection 2019. Signal Transduct Target Ther. 2019. PMID: 31637009 Free PMC article. Review.
-
Predominant Role of Serotonin at the Hippocampal Mossy Fiber Synapse with Redundant Monoaminergic Modulation.iScience. 2020 Apr 24;23(4):101025. doi: 10.1016/j.isci.2020.101025. Epub 2020 Mar 31. iScience. 2020. PMID: 32283526 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

