Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration
- PMID: 25976647
- PMCID: PMC4506345
- DOI: 10.1038/eye.2015.64
Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration
Abstract
Purpose: To compare the impact of sustained supplementation using different macular carotenoid formulations on macular pigment (MP) and visual function in early age-related macular degeneration (AMD).
Patients and methods: Sixty-seven subjects with early AMD were randomly assigned to: Group 1 (20 mg per day lutein (L), 0.86 mg per day zeaxanthin (Z); Ultra Lutein), Group 2 (10 mg per day meso-zeaxanthin (MZ), 10 mg per day L, 2 mg per day Z; Macushield; Macuhealth), Group 3 (17 mg per day MZ, 3 mg per day L, 2 mg per day Z). MP was measured using customised heterochromatic flicker photometry and visual function was assessed by measuring contrast sensitivity (CS) and best-corrected visual acuity (BCVA). AMD was graded using the Wisconsin Age-Related Maculopathy Grading System (AREDS 11-step severity scale).
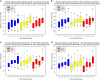
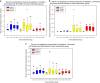
Results: At 3 years, a significant increase in MP from baseline was observed in all groups at each eccentricity (P<0.05), except at 1.75° in Group 1 (P=0.160). Between 24 and 36 months, significant increases in MP at each eccentricity were seen in Group 3 (P<0.05 for all), and at 0.50° in Group 2 (P<0.05), whereas no significant increases were seen in Group 1 (P>0.05 for all). At 36 months, compared with baseline, the following significant improvements (P<0.05) in CS were observed: Group 2-1.2, 6, and 9.6 cycles per degree (c.p.d.); Group 1-15.15 c.p.d.; and Group 3-6, 9.6, and 15.15 c.p.d. No significant changes in BCVA, or progression to advanced AMD, were observed.
Conclusion: In early AMD, MP can be augmented with a variety of supplements, although the inclusion of MZ may confer benefits in terms of panprofile augmentation and in terms of CS enhancement.
Figures
References
-
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. - PubMed
-
- Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. - PubMed
-
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811. - PubMed
-
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–223. - PubMed
-
- Nolan JM, Akkali MC, Loughman J, Howard AN, Beatty S. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp Eye Res. 2012;101:9–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

