Positive regulation of neocortical synapse formation by the Plexin-D1 receptor
- PMID: 25976775
- PMCID: PMC4465032
- DOI: 10.1016/j.brainres.2015.05.005
Positive regulation of neocortical synapse formation by the Plexin-D1 receptor
Abstract
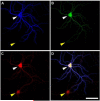
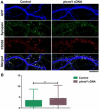
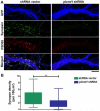
Synapse formation is a critical process during neural development and is coordinated by multiple signals. Several lines of evidence implicate the Plexin-D1 receptor in synaptogenesis. Studies have shown that Plexin-D1 signaling is involved in synaptic specificity and synapse formation in spinal cord and striatum. Expression of Plexin-D1 and its principal neural ligand, Sema3E, by neocortical neurons is temporally and spatially regulated, reaching the highest level at the time of synaptogenesis in mice. In this study, we examined the function of Plexin-D1 in synapse formation by primary neocortical neurons in vitro. A novel, automated image analysis method was developed to quantitate synapse formation under baseline conditions and with manipulation of Plexin-D1 levels. shRNA and overexpression manipulations caused opposite changes, with reduction resulting in less synapse formation, an effect distinct from that reported in the striatum. The data indicate that Plexin-D1 operates in a cell context-specific fashion, mediating different synaptogenic outcomes depending upon neuron type.
Keywords: Image analysis; In vitro; Intracortical; Mouse; Synaptogenesis.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

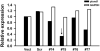
References
-
- Akins MR, Biederer T. Cell–cell interactions in synaptogenesis. Curr. Opin. Neurobiol. 2006;16:83–89. - PubMed
-
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74:975–989. - PubMed
-
- Bellon A, et al. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66:205–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

